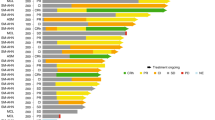
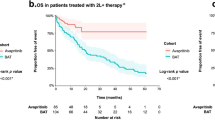
Abstract
Patients with advanced systemic mastocytosis (SM) (e.g. aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN) and mast cell leukemia (MCL)) have limited treatment options and exhibit reduced survival. Midostaurin is an oral multikinase inhibitor that inhibits D816V-mutated KIT, a primary driver of SM pathogenesis. We conducted a phase II trial of midostaurin 100 mg twice daily, administered as 28-day cycles, in 26 patients (ASM, n=3; SM-AHN, n= 17; MCL, n=6) with at least one sign of organ damage. During the first 12 cycles, the overall response rate was 69% (major/partial response: 50/19%) with clinical benefit in all advanced SM variants. With ongoing therapy, 2 patients achieved a complete remission of their SM. Midostaurin produced a ⩾50% reduction in bone marrow mast cell burden and serum tryptase level in 68% and 46% of patients, respectively. Median overall survival for the entire cohort was 40 months, and 18.5 months for MCL patients. Low-grade gastrointestinal side effects were common and manageable with antiemetics. The most frequent grade 3/4 nonhematologic and hematologic toxicities were asymptomatic hyperlipasemia (15%) and anemia (12%). With median follow-up of 10 years, no unexpected toxicities emerged. These data establish the durable activity and tolerability of midostaurin in advanced SM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L et alMastocytosis. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al(eds) WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. International Agency for Research and Cancer (IARC): Lyon, 2008; pp 54–63.
Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 2001; 25: 603–625.
Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood 2009; 113: 5727–5736.
Pardanani A . Systemic mastocytosis in adults: 2017 update on diagnosis, risk, stratification, and management. Am J Hematol 2016; 91: 1146–1159.
Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res 2003; 27: 635–641.
Sperr WR, Horny HP, Valent P . Spectrum of associated clonal hematologic non-mast cell lineage disorders occurring in patients with systemic mastocytosis. Int Arch Allergy Immunol 2002; 127: 140–142.
Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G . Mast cell leukemia. Blood 2013; 121: 1285–1295.
Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus report. Ann Oncol 2014; 25: 691–700.
Delaporte E, Piérard E, Wolthers BG, Desreumaux P, Janin A, Cortot A et al. Interferon-alpha in combination with corticosteroids improves systemic mast cell disease. Br J Dermatol 1995; 132: 479–482.
Hauswirth AW, Simonitsch-Klupp I, Uffmann M, Koller E, Sperr WR, Lechner K et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res 2004; 28: 249 257.
Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A . Cytoreductive therapy in 108 adults with systemic mastocytosis: outcome analysis and response prediction during treatment with interferon-alpha, hydroxyurea, imatinib mesylate or 2-chlorodeoxy-adenosine. Am J Hematol 2009; 84: 790–794.
Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van’t Wout JW, Verhoef G, Gerrits WB et al. Cladribine therapy for systemic mastocytosis. Blood 2003; 102: 4270–4276.
Tefferi A, Li CY, Butterfield JH, Hoagland HC . Treatment of systemic mast-cell disease with cladribine. N Engl J Med 2001; 344: 307–309.
Pardanani A, Hoffbrand AV, Butterfield JH, Tefferi A . Treatment of systemic mast cell disease with 2-chlorodeoxyadenosine. Leuk Res 2004; 28: 127–131.
Barete S, Lortholary O, Damaj G, Hirsch I, Chandesris MO, Elie C et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood 2015; 126: 1009–1016.
Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood 2006; 108: 2366–2372.
Kristensen T, Vestergaard H, Møller MB . Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagn 2011; 13: 180–188.
Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia 2015; 29: 1223–1232.
Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A et al. PKC412–a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des 2000; 15: 17–28.
Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD et al. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood 2005; 106: 721–724.
Gotlib J, Berube C, Growney JD, Chen CC, George TI, Williams C et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood 2005; 106: 2865–2870.
Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000; 96: 3671–3674.
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108: 419–425.
Simon R . Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas AD et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Gotlib J, Kluin-Nelemens HC, George TI, Akin C, Sotlar K, Hermine O et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med 2016; 374: 2530–2541.
van Anrooij B, Kluin-Nelemans JC, Safy M, Flokstra-de Blok BM, Oude Elberink JN . Patient-reported disease-specific quality-of-life and symptom severity in systemic mastocytosis. Allergy 2016; 71: 1585–1593.
Ware JE, Kosinski M, Keller SD . A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233.
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994; 30A: 1326–1336.
Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P . Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy 2009; 39: 1711–1720.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH et al. The c KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood 2002; 100: 1068–1071.
Vega-Ruiz A, Cortes JE, Sever M, Manshouri T, Quintás-Cardama A, Luthra R et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk Res 2009; 33: 1481–1484.
Pagano L, Valentini CG, Caira M, Rondoni M, Van Lint MT, Candoni A et al. Advanced mast cell disease: an Italian hematological multicenter experience. Int J Hematol 2008; 88: 483–488.
Verstovsek S, Tefferi A, Cortes J, O’Brien S, Garcia-Manero G, Pardanani A et al. Phase II study of dasatinib in Philadelphia chromosome-negative acute and chronic myeloid diseases, including systemic mastocytosis. Clin Cancer Res 2008; 14: 3906–3915.
Hochhaus A, Baccarani M, Giles FJ, le Coutre PD, Müller MC, Reiter A et al. Nilotinib in patients with systemic mastocytosis: analysis of the phase 2, open-label, single-arm nilotinib registration study. J Cancer Res Clin Oncol 2015; 141: 2047–2060.
Lortholary O, Chandesris MO, Livideanu CB, Paul C, Guillet G, Jassem E et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomized, placebo-controlled, phase 3 study. Lancet 2017; 389: 612–620.
Valent P, Sotlar K, Sperr WR, Reiter A, Arock M, Horny HP . Chronic mast cell leukemia: a novel leukemia-variant with distinct morphological and clinical features. Leuk Res 2015; 39: 1–5.
Valent P, Berger J, Cerny-Reiterer S, Peter B, Eisenwort G, Hoermann G et al. Chronic mast cell leukemia (MCL) with KIT S476I: a rare entity defined by leukemic expansion of mature mast cells and absence of organ damage. Ann Hematol 2015; 94: 223–231.
Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol 2014; 32: 3264–3274.
Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 2013; 122: 2460–2466.
Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia 2016; 30: 136–143.
Jawhar M, Schwaab J, Meggendorfer M, Naumann N, Horny HP, Sotlar K et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica 2017; 102: 1035–1043.
Jawhar M, Schwaab J, Naumann N, Horny HP, Sotlar K, Haferlach T et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood 2017; 130: 137–145.
Acknowledgements
We thank the patients and their families for their dedication to the study. We express gratitude to the Charles and Ann Johnson Foundation (to JG) and the European Competency Network in Mastocytosis (ECNM) for their support of mastocytosis research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr DeAngelo/Dana Farber Cancer Institute has received funding to conduct the current trial; he has also received funding to conduct the trial of BLU-285 (Blueprint Medicines) in advanced SM. Dr George served on the Study Steering Committee for the Novartis-sponsored global trial and she has served on an advisory board and received honoraria from Novartis. She has also received honoraria from Blueprint Medicine and research funding from Allakos, Inc. The University of New Mexico has received funding for Dr George’s participation in the Novartis-sponsored global trial of midostaurin in advanced SM, the Seattle Genetics-sponsored trial of brentuximab vedotin in advanced SM and the Blueprint Medicine-sponsored trial of BLU-285 in advanced SM. Dr Graubert/Washington University received funding to conduct the current trial. Dr Gotlib served as Chairman of the Study Steering Committee for the Novartis-sponsored global trial of midostaurin in advanced SM, received funding to conduct this trial and the global trial and he has served on an advisory board and received honoraria from Novartis. Stanford has also received funding for Dr Gotlib’s participation in the following trials in advanced SM: BLU-285 (Blueprint Medicines), brentuximab vedotin (Seattle Genetics) and ibrutinib (Pharmacyclics).
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
DeAngelo, D., George, T., Linder, A. et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia 32, 470–478 (2018). https://doi.org/10.1038/leu.2017.234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.234
This article is cited by
-
Allogeneic Hematopoietic Cell Transplantation in Advanced Systemic Mastocytosis: A retrospective analysis of the DRST and GREM registries
Leukemia (2024)
-
SETD2 non genomic loss of function in advanced systemic mastocytosis is mediated by an Aurora kinase A/MDM2 axis and can be therapeutically targeted
Biomarker Research (2023)
-
Diagnostik und Therapie der systemischen Mastozytose
best practice onkologie (2023)
-
Diagnostik und Therapie der systemischen Mastozytose
Die Onkologie (2023)
-
Response and resistance to cladribine in patients with advanced systemic mastocytosis: a registry-based analysis
Annals of Hematology (2023)