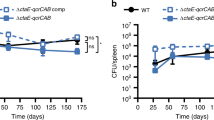
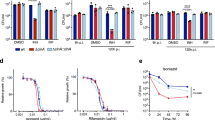
Abstract
Mycobacterium tuberculosis is a natural mutant in oxyR, a close homolog of the central regulator of peroxide stress response in enteric bacteria. Inactivation of oxyR is specific for M. tuberculosis and other members of the M. tuberculosis complex. This phenomenon appears as a paradox due to the ability of this organism to parasitize host macrophages, in which the ingested organisms are likely to be exposed to reactive oxygen intermediates. However, the surprising finding that M. tuberculosis has multiple deletions, nonsense and frameshift mutations in oxyR may help explain the exceptionally high sensitivity of M. tuberculosis to the potent antituberculosis agent isoniazid. One of the genes affected by oxyR lesions, ahpC (encoding an alkylhydroperoxide reductase) may determine the intrinsic sensitivity of mycobacteria to isoniazid.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kochi, A. 1991. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle 72: 1–6.
Bloom, B.R. and Murray, C.J.L. 1994. Tuberculosis: commentary on a reemergent killer. Science 257: 1055–1064.
Colston, M.J. and Davis, E.O. 1994. The ins and outs of protein splicing elements. Mol. Microbiol. 12: 359–363.
Dannenberg, A.M.J. and Rook, G.A.W. 1994. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication, pp. 459–483 in Tuberculosis: Pathogenesis, protection and control. Bloom, B.R. (ed.). ASM Press, Washington, D.C.
Fenton, M.J. and Vermeulen, M.W. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64: 683–690.
Jacobs, W.R., Kalpana, G.V., Cirrilo, J.D., Pascopella, L., Snapper, S.B., Udani, R.A., Jones, W., Barletta, R.G. and Bloom, B.R. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204: 537–555.
Musser, J.M. 1995. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8: 496–514.
Russell, D.G. 1995. Mycobacterium and Leishmania: stowaways in the endoso-mal network. Trends Cell. Biol. 5: 125–128.
Young, D.B. and Cole, S.T. 1993. Leprosy, tuberculosis, and the new genetics. J. Bacteriol. 175: 1–6.
Zhang, Y. and Young, D.B. 1993. Molecular mechanisms of isoniazid: a drug at the front line of tuberculosis control. Trends Microbiol. 1: 109–113.
Deretic, V., Philipp, W., Dhandayuthapani, S., Mudd, M.H., Curcic, R., Garbe, T. et al. 1995. Myobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol. Microbiol. 17: 889–900.
Dhandayuthapani, S., Via, I.E., Thomas, C.A., Horowitz, P.M., Deretic, D., Deretic, V. 1995. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol. Microbiol. 17: 901–912.
Dhandayuthapani, S., Zhang, Y., Mudd, M.H. and Deretic, V. Oxidative stress response and its role in sensitivity to isonicotinic acid hydrazide in Mycobacterium species: characterization and induclbility of ahpC by peroxides in M. smegmatis and lack of expression in M. aurum and M. tuberculosis . J. Bacteriol. 178: 12.
Sherman, D.R., Sabo, P.J., Mickey, M.J., Arain, T.M., Mahairas, G.G., Yuan, Y. et al. 1995. Disparate resposes to oxidative stress in a saprophytic and pathogenic mycobacteria. Proc. Natl. Acad. Sci. USA 92: 6625–6629.
Winder, F.G. 1982. Mode of action of the animycobacteral agents and associated aspects of the molecular biology of the mycobacteriaa, pp. 354–438 in The Biology of the Mycobacteria. Ratledge, C. and Stanford, J.S. (eds.). Academic Press, London.
Banerjee, A., Dubnau, E., Quemard, A., Balasubramanian, V., Urn, K.S., Wilson, T. et al. 1994. inha, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis . Science 263: 227–230.
Dessen, A., Quemard, A., Blanchard, J.S., Jacobs, W.R. and Sacchettini, J.C. 1995. Crystal structure and function of the isoniazid target of Mycobacterium tuberculosis . Science 267: 1638–1641.
Johnsson, K., King, D.S. and Schultz, P.G. 1995. Studies on the mechanism of action of isoniazid and ethionamide in the chemotherapy of tuberculosis. J. Am. Chem. Soc. 117: 5009–5010
Youatt, J. 1969. A review of the action of isoniazid. Am. Rev. Respir. Dis. 99: 729–749.
Gayathri-Devi, B., Shaila, M.S., Ramakrishnan, T. and Gopinathan, K.P. 1975. The purification and properties of peroxidase in Mycobacterium tuberculosis H37Rv and its possible role in the mechanism of action of isonicotinic acid hydrazide. Biochem. J. 149: 187–197.
Bekierkunst, A. 1996. Nicotinamide-adenine dinucleotide exposed to isoniazad. Science 152: 525–526.
Kasarov, L.B. and Moat, A.G. 1972. Metabolism of nicotinamide adenine dinucleotide in human and bovine strains of Mycobacterium tuberculosis . J. Bacteriol. 110: 600–603.
Kruger-Thiemer, E. 1958. Isonicotinic acid hypothesis of the antituberculous action of isoniazid. Am. Rev. Tuberc. 77: 364–367.
Seydel, J., Schaper, K.-J., Wempe, E. and Cordes, H.R. 1976. Mode of action and quantitative structure-activity correlations of tuberculostatic drugs of the isonicotinic acid hydrazide type. J. Med. Chem. 19: 483–492.
Herman, D.J. and Weber, M.M. 1980. Isonizaid interaction with tyrosine as a possible mode of action of the drug in mycobacteria. Antimicrob. Agents Chemother. 17: 170–178.
Long, E.R. 1958. pp. 362–388 in The Chemistry and Chemotherapy of Tuberculosis. Balliere, Tindall & Cox, Ltd., London.
Quemard, A., Lacaveand, C. and Laneelle, G. 1991. Isoniazid inhibition of mycolic acid synthesis by cell extracts of sensitive and resistant strains of Mycobacterium aurum . Antibicrob. Agents Chemother. 35: 1035–1039.
Winder, F.G. 1960. Catalase and peroxidase in Mycobacteria. Am. Rev. Respir. Dis. 81: 68–78.
Shoeb, H.A., Bowman, B.U., Ottolenghi, A.C. and Merola, A.J. 1985. Enzymatic and nonenzymatic superoxide-generating reactions of isoniazid. Antimicrob. Agents Chemother. 27: 408–412.
Shoeb, H.A., Bowman, B.U., Ottolenghi, A.C. and Merola, A.J. 1985. Evidence for the generation of active oxygen by isoniazid treatment of extracts of Mycobacterium tuberculosis H37Ra . Antimicrob, Agents Chemother. 27: 404–407.
Shoeb, H.A., Bowman, B.U., Ottolenghi, A.C. and Merola, A.J. 1985. Peroxidase-mediated oxidation of isoniazid. Antimicrob. Agents Chemother. 27: 399–403.
Zinner, K., Vidgal, C.C.C., Duran, N. and Cilento, G. 1977. Oxidation of isonicotininc acid hydrazide by the peroxidase system. Arch. Biochem. Biophys. 180: 452–458.
Christman, M.F., Morgan, R.W., Jacobson, F.S. and Ames, B.N. 1985. Positive control of a regulation for defences against oxidative stress and some heat-shock proteins in Salmonella typhimurium . Cell 41: 753–762.
Christman, M.F., Storz, G. and Ames, B.N. 1989. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. USA 86: 3484–3488.
Toledano, M.B., Kullik, I., Trinh, F., Baird, P.T., Schneider, T.D. and Storz, G. 1994. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78: 897–909.
Farr, S.B. and Kogoma, T. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium . Microbiol. Rev. 55: 561–585.
Middlebrook, G. and Cohn, M.L. 1953. Some observations on the pathogenicity of isoniazid-resistant variants of tubercle bacilli. Science 118: 297–299.
Middlebrook, G. 1954. Isoniazid-resistance and catalase activity of tubercle bacilli. Am. Rev. Tuberc. 69: 471–472.
Zhang, Y., Heym, B., Allen, B., Young, D. and Cote, S.T. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis . Nature 358: 591–593.
Heym, B., Alzari, P.M., Honors, N. and Cole, S.T. 1995. Missense mutations in the catalase-peroxidase gene, katG are associated with isoniazid resistance in Mycobacterium tuberculosis . Mol. Microbiol. 15: 235–245.
Cockerill, F.R., Uhl, J.R., Temesgen, Z., Zhang, Y., Stockman, L., Roberts, G.D. et al 1995. Rapid identification of a point mutation of the Mycobacterium tuberculosis catalase-peroxidase associated with isoniazid resistance. J. Infect. Dis. 171: 240–245
Rouse, D.A. and Morris, S.L. 1995. Molecular mechanisms of isoniazid resistance in Mycobacterium tuberculosis and Mycobacterium bovis . Infect. Immun. 63: 1427–1433.
Pretorius, G.S., Hekten, R.D., Siigel, R., Eisenach, K.D. and Victor, T.C. 1995. Mutations in katG gene sequences in isoniazid-resistant clinical isolates of Mycobacterium tuberculosis are rare. Antimicrob. Agents Chemother. 39: 2276–2281.
Rouse, D.A., Li, Z., Bai, G.-H. and Morris, S.L. 1995. Characterization of the katG and inhA genes of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis . Antimicrob. Agents Chemother. 39: 2472–2477.
Bergh, S. and Cole, S.T. 1994. MycDB: an integrated mycobacterial database. Mol. Microbiol. 12: 534–574.
Zhang, Y., Garbe, T. and Young, D. 1993. Transformation with katG restores isoniazid-sensitlvity in Mycobacterium tuberculosis isolates resistant to a range of drug concentrations. Mol. Microbiol. 8: 521–524.
Milano, A., De Rossi, E., Gusberti, L., Heym, B., Marone, P. and Riccardi, G. 1996. The katE gene, which encodes the catalase HPII of Mycobacterium avium . Mol. Microbiol. 19: 113–123.
Rosner, J.L. 1993. Susceptibilities of oxyR regulon mutants of Escherichia coli and Salmonella typhimurium to isoniazid. Antimicrob. Agents Chemother. 37: 2251–2253.
Yamaguchi, R., Mutsuo, K., Yamazaki, A., Takahashi, M., Fukusawa, Y., Wada, M. and Abe, C. 1992. Cloning and expression of the gene for the Avi-3 antigen of Mycobacterium avium and mapping of its epitopes. Infect. Immun. 60: 1210–1216.
Schell, M.A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47: 597–626.
Jacobson, F.S., Morgan, R.W., Christman, M.F. and Ames, B.N. 1989. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. J. Biol. Chem. 264: 1488–1496.
Storz, G.S., Christman, M.F., Sies, H. and Ames, B.N. 1987. Spontanous mutagenesis and oxidative damage to DNA in Salmonella typhimurium . Proc. Natl. Acad. Sci. USA 84: 8917–8921.
Chae, H.Z., Robinson, K., Poole, L.B., Church, G., Storz, G. and Rhee, S.G. 1994. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 91: 7017–7021).
Zhang, Y., Dhandayuthapani, S. and Deretic, V. 1996. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc. Natl. Acad. Sci. USA. In press.
Le Lirzin, M.J.N., Vivien, J.N., Lepeuple, A., Thibier, R. and Pretet, C. 1971. Rapid microbiological estimation of serum isoniazid. Rev. Tuberc. Pneumol. 35: 350–356.
Heym, B. and Cole, S.T. 1992. Isolation and characterization of isoniazid-resistant mutants of Mycobacterium smegmatis and M. aurum . Res. Microbiol. 143: 721–730.
Wilson, T.M. and Collins, D.M. 1996. aphC, a gene involved in isoniazid resistance of the Mycobacterium tuberculosis complex. Mol. Microbiol. 19: 1025–1034.
Sherman, D.R., Mdluli, K., Hickey, M.J., Arain, T.M., Morris, S.L., Barry, C.E. and Stover, C.K. 1996. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis . Science 272: 1641–1643.
Sreevantsan, S., Zhang, Y., Deretic, V. and Musser, J.M. Analysis of the oxyR-ahpC region in isoniazid-resistant and susceptible Mycobacterium tuberculosis complex organisms recovered from diseased humans and animals in diverse localities. Submitted.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deretic, V., Pagán-Ramos, E., Zhang, Y. et al. The extreme sensitivity of Mycobacterium tuberculosis to the front-line antituberculosis drug isoniazid. Nat Biotechnol 14, 1557–1561 (1996). https://doi.org/10.1038/nbt1196-1557
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1196-1557
This article is cited by
-
Design, synthesis, and evaluation of isoniazid derivatives acting as potent anti-inflammatory and anthelmintic agents via Betti reaction
Medicinal Chemistry Research (2014)
-
Microbial transformation of azaarenes and potential uses in pharmaceutical synthesis
Applied Microbiology and Biotechnology (2012)
-
Isolation of oxidative stress response mutants in Escherichia coli for their use as a genetic screen to identify new anti-mycobacterials as functional analogues of isoniazid
World Journal of Microbiology and Biotechnology (2006)