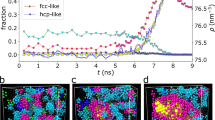
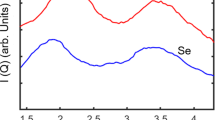
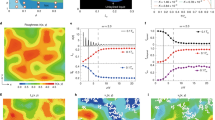
Abstract
Crystallization is a fundamental process in materials science, providing the primary route for the realization of a wide range of new materials. Crystallization rates are also considered to be useful probes of glass-forming ability1,2,3. At the microscopic level, crystallization is described by the classical crystal nucleation and growth theories4,5, yet in general solid formation is a far more complex process. In particular, the observation of apparently different crystal growth regimes in many binary liquid mixtures greatly challenges our understanding of crystallization1,6,7,8,9,10,11,12. Here, we study by experiments, theory and computer simulations the crystallization of supercooled mixtures of argon and krypton, showing that crystal growth rates in these systems can be reconciled with existing crystal growth models only by explicitly accounting for the non-ideality of the mixtures. Our results highlight the importance of thermodynamic aspects in describing the crystal growth kinetics, providing a substantial step towards a more sophisticated theory of crystal growth.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used during the current study are available from the corresponding author on reasonable request.
Change history
03 March 2020
In the version of this Article originally published, the Δ in ΔSm and ΔG in the paragraph after equation (1) should have been roman; the x in (1 − x) before equation (12) should have been italic; and ‘measurement’, ‘thermodynamic’ and ‘vibrational’ in refs. 35, 36 and 39, respectively, should have been capitalized. These have now been updated.
References
Tang, C. & Harrowell, P. Anomalously slow crystal growth of the glass-forming alloy CuZr. Nat. Mater. 12, 507–511 (2013).
Orava, J. & Greer, A. L. Fast and slow crystal growth kinetics in glass-forming melts. J. Chem. Phys. 140, 214504 (2014).
Orava, J. & Greer, A. L. Fast crystal growth in glass-forming liquids. J. Non-Cryst. Solids 451, 94–100 (2016).
Kelton, K. F. & Greer, A. L. Nucleation in Condensed Matter (Elsevier, 2010).
Jackson, K. A. Kinetic Processes (Wiley, 2004).
Spaepen, F. & Lin, C.J. in Amorphous Metals and Non-Equilibrium Processing (ed. von Allmen, M.) 65–72 (Les Ulis, Les Editions de Physique, 1984).
Kerrache, A., Horbach, J. & Binder, K. Molecular-dynamics computer simulation of crystal growth and melting in Al50 Ni50. Europhys. Lett. 81, 58001 (2008).
Stipp, A. & Palberg, T. Crystal growth kinetics in binary mixtures of model charged sphere colloids. Phil. Mag. Lett. 87, 899–908 (2007).
Wang, Q. et al. Diffusion-controlled crystal growth in deeply undercooled Zr50 Cu50 melt on approaching the glass transition. Phys. Rev. B. 83, 014202 (2011).
Fang, T., Wang, L. & Qi, Y. Solid-liquid interface growth of Cu50 Ni50 under deep undercoolings. Phys. Chem. Liquids 52, 342–348 (2013).
Yan, X. Q. & Lü, Y. J. Mechanism of abnormally slow crystal growth of CuZr alloy. J. Chem. Phys. 143, 164503 (2015).
Sun, Y. et al. Effects of dopants on the glass forming ability in Al-based metallic alloy. Phys. Rev. Mater. 3, 023404 (2019).
Frenkel, Y. The Kinetic Theory of Liquids (Oxford Univ. Press, 1946).
Broughton, J. Q., Gilmer, G. H. & Jackson, K. A. Crystallization rates of a Lennard-Jones liquid. Phys. Rev. Lett. 49, 1496–1500 (1982).
Hawken, A., Sun, G. & Harrowell, P. Role of interfacial inherent structures in the fast crystal growth from molten salts and metal. Phys. Rev. Mater. 3, 043401 (2019).
Sun, G., Xu, J. & Harrowell, P. The mechanism of the ultrafast crystal growth of pure metals from their melts. Nat. Mater. 17, 881–886 (2018).
Burke, E., Broughton, J. Q. & Gilmer, G. H. Crystallization of FCC (111) and (100) crystal-melt interfaces: a comparison by molecular dynamics for the Lennard-Jones system. J. Chem. Phys. 89, 1030–1041 (1988).
Heastie, R. Properties of solid and liquid solutions of argon and krypton. Proc. Phys. Soc. 73, 490–500 (1959).
Kühnel, M. et al. Time-resolved study of crystallization in deeply cooled liquid parahydrogen. Phys. Rev. Lett. 106, 245301 (2011).
Grisenti, R. E., Kalinin, A., Goy, C. & Schottelius, A. Evaporating laminar microjets for studies of rapidly evolving structural transformations in supercooled liquids. Adv. Phys. X 3, 1418183 (2018).
Pollack, G. L. The solid state of rare gases. Rev. Mod. Phys. 36, 748–791 (1964).
Cahn, J. W. Transformation kinetics during continuous cooling. Acta Metallurgica 4, 572–575 (1956).
Williams, S. R., Royall, C. P. & Bryant, G. Crystallization of dense binary hard-sphere mixtures with marginal size ratio. Phys. Rev. Lett. 100, 225502 (2008).
Kühnel, M. et al. Observation of crystallization slowdown in supercooled parahydrogen and orthodeuterium quantum liquid mixtures. Phys. Rev. B. 89, 180201(R) (2014).
Taylor, R. & Krishna, R. Multicomponent Mass Transfer (Wiley, 1993).
Rowlinson, J. S. & Swinton, F. L. Liquids and Liquid Mixtures (Butterworth & Co, 1982).
Darken, L. S. Diffusion, mobility and their interrelation through free energy in binary metallic systems. Trans. Am. Inst. Min. Metall. Eng. 175, 184–201 (1948).
Turchanin, M. A., Agraval, P. G. & Abdulov, A. R. Phase equilibria and thermodynamics of binary copper systems with 3d-metals. VI The copper-nickel system. Powder Metall. Met. Ceram. 45, 467–477 (2007).
Willnecker, R., Herlach, D. M. & Feuerbacher, B. Evidence of nonequilibrium processes in rapid solidification of undercooled metals. Phys. Rev. Lett. 62, 2707–2710 (1989).
Algoso, P. R., Hofmeister, W. H. & Bayuzick, R. J. Solidification velocity of undercooled Ni-Cu alloys. Acta Mater. 51, 4307–4318 (2003).
Ferreira, A. G. M. & Lobo, L. Q. The sublimation of argon, krypton, and xenon. J. Chem. Thermodynamics 40, 1621–1626 (2008).
Kovalenko, S. I., Indan, E. I. & Khudoteplaya, A. A. An electron-diffraction study of thin films of the binary mixtures of rare gases. Phys. Stat. Sol. 13, 235–240 (1972).
Vignes, A. Diffusion in binary solutions. Ind. Eng. Chem. Fundamentals 5, 189–199 (1966).
Cullinan, H. T. Jr. Concentration dependence of the binary diffusion coefficient. Ind. Eng. Chem. Fundamentals 5, 281–283 (1966).
Naghizadeh, J. & Rice, S. A. Kinetic theory of dense fluids. X. Measurement and interpretation of self-diffusion in liquid Ar, Kr, Xe, and CH4. J. Chem. Phys. 36, 2710–2720 (1962).
Flubacher, P., Leadbetter, A. J. & Morrison, J. A. A low temperature adiabatic calorimeter for condensed substances. Thermodynamic properties of argon. Proc. Phys. Soc. 78, 1449–1461 (1961).
Gladun, C. & Menzel, F. The specific heat and some other thermodynamic properties of liquid krypton. Cryogenics 10, 210–213 (1970).
Gladun, C. The specific heat of liquid argon. Cryogenics 11, 205–209 (1971).
Beaumont, R. H., Chihara, H. & Morrison, J. A. Thermodynamic properties of krypton. Vibrational and other properties of solid argon and solid krypton. Proc. Phys. Soc. 78, 1462–1481 (1961).
Davies, R. H., Duncan, A. G., Saville, G. & Staveley, L. A. K. Thermodynamics of liquid mixtures of argon and krypton. Trans. Faraday Soc. 63, 855–869 (1967).
Fender, B. E. F. & Halsey, G. D. Jr. Solid solution of argon and krypton; refined measurements. J. Chem. Phys. 42, 127–131 (1965).
Steinhardt, P., Nelson, D. R. & Ronchetti, M. Bond-orientational order in liquids and glasses. Phys. Rev. B. 28, 784–805 (1983).
Acknowledgements
We acknowledge financial support from the Bundesministerium für Bildung und Forschung (grant no. 05K13RF5). We acknowledge the CINECA award nos. LISA-PUMAS (2016), IscraC-GLEMD (2017) and IscraB-MEMETICO (2018) for the availability of high-performance computing resources and support. We acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were carried out at PETRA III and we thank S. Roth for the unlimited support during the experiments at the beamline P03.
Author information
Authors and Affiliations
Contributions
A.S. and F.M. contributed equally to this work. R.E.G. conceived the experiment. A.S. designed and assembled the experimental setup. A.S., F.M., A.K., B.B., A.R., C.G., J.M., N.P., M.R., F.T., J.M.F., T.A.E. and R.E.G. performed the experiments. A.S. and R.E.G. analysed the experimental data. R.E.G. performed the theoretical crystal growth rate calculations. F.M. and D.E.G. conceived and carried out the molecular dynamics simulations. F.M., D.E.G. and R.E.G. wrote the paper, with contributions from all authors
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Schottelius, A., Mambretti, F., Kalinin, A. et al. Crystal growth rates in supercooled atomic liquid mixtures. Nat. Mater. 19, 512–516 (2020). https://doi.org/10.1038/s41563-020-0613-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0613-z