Abstract
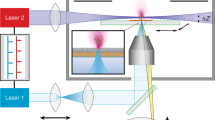
Mass spectrometry imaging (MSI) enables label-free spatial mapping of hundreds of biomolecules in tissue sections. This capability provides valuable information on tissue heterogeneity that is difficult to obtain using population-averaged assays. Despite substantial developments in both instrumentation and methodology, MSI of tissue samples at single-cell resolution remains challenging. Herein, we describe a protocol for robust imaging of tissue sections with a high (better than 10-μm) spatial resolution using nanospray desorption electrospray ionization (nano-DESI) mass spectrometry, an ambient ionization technique that does not require sample pretreatment before analysis. In this protocol, mouse uterine tissue is used as a model system to illustrate both the workflow and data obtained in these experiments. We provide a detailed description of the nano-DESI MSI platform, fabrication of the nano-DESI and shear force probes, shear force microscopy experiments, spectral acquisition, and data processing. A properly trained researcher (e.g., technician, graduate student, or postdoc) can complete all the steps from probe fabrication to data acquisition and processing within a single day. We also describe a new strategy for acquiring both positive- and negative-mode imaging data in the same experiment. This is achieved by alternating between positive and negative data acquisition modes during consecutive line scans. Using our imaging approach, hundreds of high-quality ion images were obtained from a single uterine section. This protocol enables sensitive and quantitative imaging of lipids and metabolites in heterogeneous tissue sections with high spatial resolution, which is critical to understanding biochemical processes occurring in biological tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data presented in this study are available from the corresponding author upon reasonable request.
Code availability
The LabVIEW and MSI QuickView programs are available from the corresponding author upon reasonable request.
References
Norris, J. L. & Caprioli, R. M. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem. Rev. 113, 2309–2342 (2013).
Vaysse, P.-M., Heeren, R., Porta, T. & Balluff, B. Mass spectrometry imaging for clinical research–latest developments, applications, and current limitations. Analyst 142, 2690–2712 (2017).
Chaurand, P. Imaging mass spectrometry of thin tissue sections: a decade of collective efforts. J. Proteom. 75, 4883–4892 (2012).
Wu, C., Dill, A. L., Eberlin, L. S., Cooks, R. G. & Ifa, D. R. Mass spectrometry imaging under ambient conditions. Mass Spectrom. Rev. 32, 218–243 (2013).
Laskin, J. & Lanekoff, I. Ambient mass spectrometry imaging using direct liquid extraction techniques. Anal. Chem. 88, 52–73 (2015).
Feider, C. L., Krieger, A., DeHoog, R. J. & Eberlin, L. S. Ambient ionization mass spectrometry: recent developments and applications. Anal. Chem. 91, 4266–4290 (2019).
Rubakhin, S. S., Jurchen, J. C., Monroe, E. B. & Sweedler, J. V. Imaging mass spectrometry: fundamentals and applications to drug discovery. Drug Discov. Today 10, 823–837 (2005).
Greer, T., Sturm, R. & Li, L. Mass spectrometry imaging for drugs and metabolites. J. Proteom. 74, 2617–2631 (2011).
Kompauer, M., Heiles, S. & Spengler, B. Atmospheric pressure MALDI mass spectrometry imaging of tissues and cells at 1.4-μm lateral resolution. Nat. Methods 14, 90–96 (2017).
Zavalin, A. et al. Direct imaging of single cells and tissue at sub-cellular spatial resolution using transmission geometry MALDI MS. J. Mass Spectrom. 47, 1473–1481 (2012).
Laskin, J., Heath, B. S., Roach, P. J., Cazares, L. & Semmes, O. J. Tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 84, 141–148 (2011).
Lanekoff, I. et al. Automated platform for high-resolution tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 84, 8351–8356 (2012).
Lanekoff, I. et al. Imaging nicotine in rat brain tissue by use of nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 85, 882–889 (2013).
Lanekoff, I., Thomas, M. & Laskin, J. Shotgun approach for quantitative imaging of phospholipids using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 86, 1872–1880 (2014).
Nguyen, S. N. et al. Towards high-resolution tissue imaging using nanospray desorption electrospray ionization mass spectrometry coupled to shear force microscopy. J. Am. Soc. Mass. Spectrom. 29, 316–322 (2017).
Yin, R. et al. High spatial resolution imaging of mouse pancreatic islets using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 90, 6548–6555 (2018).
Bergman, H.-M., Lundin, E., Andersson, M. & Lanekoff, I. Quantitative mass spectrometry imaging of small-molecule neurotransmitters in rat brain tissue sections using nanospray desorption electrospray ionization. Analyst 141, 3686–3695 (2016).
Duncan, K. D. & Lanekoff, I. Oversampling to improve spatial resolution for liquid extraction mass spectrometry imaging. Anal. Chem. 90, 2451–2455 (2018).
Lanekoff, I. et al. Trp53 deficient mice predisposed to preterm birth display region-specific lipid alterations at the embryo implantation site. Sci. Rep. 6, 33023 (2016).
Nguyen, S. N., Liyu, A. V., Chu, R. K., Anderton, C. R. & Laskin, J. Constant-distance mode nanospray desorption electrospray ionization mass spectrometry imaging of biological samples with complex topography. Anal. Chem. 89, 1131–1137 (2016).
Lanekoff, I. & Laskin, J. Imaging of lipids and metabolites using nanospray desorption electrospray ionization mass spectrometry. Methods Mol. Biol. 1203, 99–106 (2015).
Ballesteros Katemann, B., Schulte, A. & Schuhmann, W. Constant-distance mode scanning electrochemical microscopy (SECM)—part I: adaptation of a non-optical shear-force-based positioning mode for SECM tips. Chem. Eur. J. 9, 2025–2033 (2003).
Ndobo-Epoy, J. P., Lesniewska, E. & Guicquero, J. P. Shear force microscopy with a nanoscale resolution. Ultramicroscopy 103, 229–236 (2005).
Wang, H. & Dey, S. K. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199 (2006).
Heeren, R. M., Smith, D. F., Stauber, J., Kukrer-Kaletas, B. & MacAleese, L. Imaging mass spectrometry: hype or hope? J. Am. Soc. Mass Spectrom. 20, 1006–1014 (2009).
Hankin, J. A. & Murphy, R. C. Relationship between MALDI IMS intensity and measured quantity of selected phospholipids in rat brain sections. Anal. Chem. 82, 8476–8484 (2010).
Lanekoff, I., Stevens, S. L., Stenzel-Poore, M. P. & Laskin, J. Matrix effects in biological mass spectrometry imaging: identification and compensation. Analyst 139, 3528–3532 (2014).
Duncan, K. D., Bergman, H.-M. & Lanekoff, I. A pneumatically assisted nanospray desorption electrospray ionization source for increased solvent versatility and enhanced metabolite detection from tissue. Analyst 142, 3424–3431 (2017).
Bodzon‐Kulakowska, A. & Suder, P. Imaging mass spectrometry: instrumentation, applications, and combination with other visualization techniques. Mass Spectrom. Rev. 35, 147–169 (2016).
Gamble, L. J. & Anderton, C. R. Secondary ion mass spectrometry imaging of tissues, cells, and microbial systems. Micros Today 24, 24–31 (2016).
Tian, H. et al. Gas cluster ion beam time-of-flight secondary ion mass spectrometry high-resolution imaging of cardiolipin speciation in the brain: identification of molecular losses after traumatic injury. Anal. Chem. 89, 4611–4619 (2017).
Tian, H. et al. Secondary-ion mass spectrometry images cardiolipins and phosphatidylethanolamines at the subcellular level. Angew. Chem. 131, 3188–3193 (2019).
Zavalin, A., Yang, J., Hayden, K., Vestal, M. & Caprioli, R. M. Tissue protein imaging at 1 μm laser spot diameter for high spatial resolution and high imaging speed using transmission geometry MALDI TOF MS. Anal. Bioanal. Chem. 407, 2337–2342 (2015).
Rao, W., Pan, N. & Yang, Z. High resolution tissue imaging using the single-probe mass spectrometry under ambient conditions. J. Am. Soc. Mass Spectrom. 26, 986–993 (2015).
Campbell, D. I., Ferreira, C. R., Eberlin, L. S. & Cooks, R. G. Improved spatial resolution in the imaging of biological tissue using desorption electrospray ionization. Anal. Bioanal. Chem. 404, 389–398 (2012).
Kim, J. Y. et al. Atmospheric pressure mass spectrometric imaging of live hippocampal tissue slices with subcellular spatial resolution. Nat. Commun. 8, 2113 (2017).
Luxembourg, S. L., Mize, T. H., McDonnell, L. A. & Heeren, R. M. High-spatial resolution mass spectrometric imaging of peptide and protein distributions on a surface. Anal. Chem. 76, 5339–5344 (2004).
Schwartz, S. A., Reyzer, M. L. & Caprioli, R. M. Direct tissue analysis using matrix‐assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J. Mass Spectrom. 38, 699–708 (2003).
Stoeckli, M., Staab, D. & Schweitzer, A. Compound and metabolite distribution measured by MALDI mass spectrometric imaging in whole-body tissue sections. Int. J. Mass Spectrom. 260, 195–202 (2007).
Nguyen, S. N. et al. Lipid coverage in nanospray desorption electrospray ionization mass spectrometry imaging of mouse lung tissues. Anal. Chem. 91, 11629–11635 (2019).
Duncan, K. D. et al. Quantitative mass spectrometry imaging of prostaglandins as silver ion adducts with nanospray desorption electrospray ionization. Anal. Chem. 90, 7246–7252 (2018).
Wu, C., Ifa, D. R., Manicke, N. E. & Cooks, R. G. Rapid, direct analysis of cholesterol by charge labeling in reactive desorption electrospray ionization. Anal. Chem. 81, 7618–7624 (2009).
Garza, K. Y. et al. Desorption electrospray ionization mass spectrometry imaging of proteins directly from biological tissue sections. Anal. Chem. 90, 7785–7789 (2018).
Hsu, C. C., Chou, P. T. & Zare, R. N. Imaging of proteins in tissue samples using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 87, 11171–11175 (2015).
Burnum, K. E. et al. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J. Lipid Res. 50, 2290–2298 (2009).
Song, H. et al. Cytosolic phospholipase A2alpha is crucial for ‘on-time’ embryo implantation that directs subsequent development. Development 129, 2879–2889 (2002).
Brash, A. R., Boeglin, W. E. & Chang, M. S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. USA 94, 6148–6152 (1997).
Lim, H. et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91, 197–208 (1997).
Walsh, S. W. Evidence for 5-hydroxyeicosatetraenoic acid (5-HETE) and leukotriene C4(LTC4) in the onset of labor. Ann. NY Acad. Sci. 622, 341–354 (1991).
Lanekoff, I. et al. Three-dimensional imaging of lipids and metabolites in tissues by nanospray desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 407, 2063–2071 (2015).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under award UG3HL145593 (HuBMAP Program, J.L. and K.E.B.-J.), the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, grant R21 HD084788 to K.E.B.-J.), and NIH grants (DA006668 and HD068524 to S.K.D.).
Author information
Authors and Affiliations
Contributions
J.L. and R.Y. developed the procedure. R.Y. performed the imaging experiments and processed the data. K.E.B.-J. and J.L. conceived the study and assisted with data analysis. X.S. and S.K.D. provided mouse uterine sections and were involved in the interpretation of the results. R.Y. and J.L. wrote the manuscript with assistance from K.E.B.-J., X.S., and S.K.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Pierre Chaurand and Livia Schiavinato Eberlin for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Nguyen, S. N. et al. J. Am. Soc. Mass Spectrom. 29, 316–322 (2018): https://doi.org/10.1007/s13361-017-1750-8
Yin, R. et al. Anal. Chem. 90, 6548–6555 (2018): https://doi.org/10.1021/acs.analchem.8b00161
Nguyen, S. N., Liyu, A. V., Chu, R. K., Anderton, C. R. & Laskin, J. Anal. Chem. 89, 1131–1137 (2016): https://doi.org/10.1021/acs.analchem.6b03293
Supplementary information
Rights and permissions
About this article
Cite this article
Yin, R., Burnum-Johnson, K.E., Sun, X. et al. High spatial resolution imaging of biological tissues using nanospray desorption electrospray ionization mass spectrometry. Nat Protoc 14, 3445–3470 (2019). https://doi.org/10.1038/s41596-019-0237-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0237-4
This article is cited by
-
A critical evaluation of ultrasensitive single-cell proteomics strategies
Analytical and Bioanalytical Chemistry (2024)
-
Regulation of β-cell death by ADP-ribosylhydrolase ARH3 via lipid signaling in insulitis
Cell Communication and Signaling (2024)
-
Automated imaging and identification of proteoforms directly from ovarian cancer tissue
Nature Communications (2023)
-
Microlensed fiber allows subcellular imaging by laser-based mass spectrometry
Nature Protocols (2023)
-
Mass spectrometry imaging for biosolids characterization to assess ecological or health risks before reuse
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.