Abstract
The theoretical optimal olfactory search strategy is to move cross-wind. Empirical evidence supporting wind-associated directionality among carnivores, however, is sparse. We examined satellite-linked telemetry movement data of adult female polar bears (Ursus maritimus) from Hudson Bay, Canada, in relation to modelled winds, in an effort to understand olfactory search for prey. In our results, the predicted cross-wind movement occurred most frequently at night during winter, the time when most hunting occurs, while downwind movement dominated during fast winds, which impede olfaction. Migration during sea ice freeze-up and break-up was also correlated with wind. A lack of orientation during summer, a period with few food resources, likely reflected reduced cross-wind search. Our findings represent the first quantitative description of anemotaxis, orientation to wind, for cross-wind search in a large carnivore. The methods are widely applicable to olfactory predators and their prey. We suggest windscapes be included as a habitat feature in habitat selection models for olfactory animals when evaluating what is considered available habitat.
Similar content being viewed by others
Introduction
Foraging efficiency, energy acquisition per unit time, is central to an animal’s fitness. Natural selection favours behaviours that maximize energy intake while minimizing foraging time1. Foraging behaviour by predators can be classified into two broad classes: ambush predation and active search predation2. For ambush predation, fitness is largely determined by habitat selection3. For active search predation, studies have expounded the significance of duration of patch use4 and prey selection5, however, research on optimal search strategies among large carnivores remains sparse6. Search strategies are especially important for success at large scales6.
Olfactory search is common for foraging carnivores7, beginning with identifying the presence of prey through odour detection, followed by prey localization8. The optimal olfactory search and localization strategies are largely dependent on the structure of odour plumes and patterns of odour dispersion8. In turbulent flow, odours are concentrated into meandering filaments, which occur at higher densities approaching the odour source9,10. Time-averaged odour concentration can be described by the Gaussian dispersion model, whereby the maximum odour concentration is along the horizontal axis in the direction of the wind, and mean concentration follows a normal distribution laterally and vertically10. Gaussian dispersion, however, does not accurately describe instantaneous odour distribution and cannot be assumed for plume-tracking at small spatiotemporal scales11. Nonetheless, Gaussian dispersal performs well for behaviours related to longer-term exposure, such as large-scale olfactory search10. During olfactory search, movement should lead to position a predator where it is most likely to encounter an odour filament. Once detected, the predator should move upwind from the location of detection to localize the source, with successful location resulting in a predation attempt or a kill. For olfactory search at large scales or in steady winds, traveling cross-wind is the optimal path for encountering an odour plume11,12. However other search strategies such as up-wind, down-wind, Lévy walk, and correlated random walk may be effective depending on patterns of odour dispersion8,13. Anemotaxis, orientation relative to wind, is well documented among insects and, more recently, among some birds, which travel cross-wind when searching for an odour and upwind when localizing the source14,15,16, however, research on olfactory search among mammalian predators is sparse17.
Polar bears (Ursus maritimus) exhibit both ambush and active search predation strategies. Most polar bear foraging is confined to the sea ice, which also serves as the prime platform for travel and mating18. When hunting their primary prey, ringed seal (Pusa hispida) and bearded seal (Erignathus barbatus), polar bears may actively search for subnivean ringed seal pupping lairs or hauled-out seals, or ambush seals surfacing at breathing holes or along the floe edge19,20,21. Ringed seal densities can range from 0.46–1.6 seals/km2 and bearded seal densities can range from 0.0036–0.0229 seals/km2 in Hudson Bay22. Ringed seal breathing holes are spaced approximately 200 m or more apart19,23 and used by only 1–2 seals19,22 because clumped distributions may increase predation risk19. Ringed seals prefer land-fast ice and large floes with leads in pack ice22. Deformation of sea ice results in ridging that reach a mean peak height of 2 m, mean width of 12 m, and account for 10–40% of sea ice volume24. Therefore, vision is less effective for polar bears to locate prey from a distance. In addition to vision, polar bears exhibit strong responses to odours and often resort to olfactory search19,20,23,25 because winds can carry odours across the complex icescape. Olfactory bulb size is correlated with home range size among carnivores7, and polar bear home ranges are disproportionately large for their body size26 further suggesting reliance on olfaction. Although detection distance is hard to estimate in mammals27, estimates for polar bears suggest they may detect seal breathing holes up to 3 km away28. Additionally, olfactory predation is presumed to underlie ringed seal haul-out behaviour: they face downwind when hauled-out, enabling them to visually detect bears approaching from downwind and detect upwind bears by scent29. While odours associated with female ringed seals and their pups are unstudied, male ringed seals are known to produce pungent odours from facials glands30. Olfaction is likely also important in polar bear reproductive behaviour; males assess the reproductive status of females through their footprints and locate females by tracking their scent31. Females with cubs, may also use olfaction to avoid males due to risk of infanticide32,33.
Olfactory search is likely dependent on a number of factors including season, time of day, wind speed, and prey distribution. Polar bear populations inhabiting regions of seasonal sea ice are on land during the ice-free summer, move onto the ice during freeze-up, remain on the sea ice during winter and spring, and return to land during break-up. During summer, without access to their primary prey, terrestrial foraging is limited primarily to berries, seaweed, vegetation, bird eggs, and carrion34,35. Because these terrestrial food sources are not as energetically dense as seal fat36,37, polar bears prioritize energy conservation over energy acquisition and minimize unnecessary movement32,36,38,39. During freeze-up, bears may favour dispersion over immediate foraging to minimize intraspecific competition or, for females with dependent young, minimize risk of predation on their cubs32,33. Late winter and spring coincide with the peak in ringed seal and bearded seal pupping, when the majority of foraging takes place and bears enter hyperphagia40. During break-up, sea ice becomes increasingly dynamic and bears may favour travelling against the drift to maintain their relative position41,42 or move to shore as the cost of travelling exceeds the benefit of foraging43,44. With respect to time of day, olfactory search likely increases during periods of reduced visibility. For example, nocturnal moths rely more on olfaction to locate flowers than diurnal moths of the same subfamily, which rely more on visual search45.
Odour filament density and distribution within a plume are affected by wind speed. In slow winds, there may be insufficient directionality to assess the source of an odour, thus, bears may move independently of wind direction. Fast winds increase turbulence intensity and may disrupt odour plumes, which can impede odor localization13,46,47. Thus, cross-wind olfactory search is expected to occur more frequently under moderate wind speeds.
We used global positioning system (GPS) telemetry location data from adult female polar bears and modelled surface windscapes to examine the significance olfaction plays in movement patterns, and test the hypothesis that bears move cross-wind during olfactory search. We specifically examined cross-wind olfactory search as our sampling rate was too low (4-hour and 30-minute fix rate) to investigate upwind movement behaviour associated with localization of the source. We predicted that the use of cross-wind olfactory search by polar bears would be more common during winter, at night, and under moderate wind speeds.
Methods
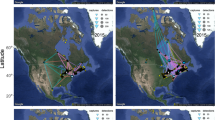
Hudson Bay, Canada is a large inland sea, which covers an area of 83*104 km2 (Fig. 1)48 and is seasonally ice-free49. From January to early May, it is covered by both fast ice (connected to shore or sea bottom) and drifting pack ice50. During break-up (early July), the motile ice drifts southeast as a consequence of the counter-clockwise gyre51 and northwesterly winds52.
Shaded area represents the western Hudson Bay (WH) polar bear subpopulation management boundary. Map was created using QGIS version 2.14 ESSEN (http://www.qgis.org/en/site/)80.
As part of a study of the population ecology of polar bears in western Hudson Bay44,53,54, polar bears were captured during the summers of 2004–2014. Bears were located and captured from helicopters55, and a sample of adult females with offspring were fitted with Argos® satellite-linked GPS collars (Telonics, Mesa, AZ). Collars were programmed to last 2 years, and had release mechanisms to drop them on a predefined date. Lone females were not collared as they were possibly pregnant and would remain in maternity dens up to seven months after collaring. Males were not collared because their neck circumference is greater than their head circumference and do not retain collars. Animal handling protocols were approved by the University of Alberta Animal Care and Use Committee for Biosciences and by the Environment Canada Prairie and Northern Region Animal Care Committee. Animal handling and collar deployments performed were in accordance with the approved protocols.
A total of 123 collars were deployed (9–15 per year); most (120) obtained one location every 4 hours, whereas 3 obtained locations every 30 minutes and were analyzed separately. The latitude and longitude coordinates were converted into Universal Trans Mercator coordinate system (NAD83 Teranet Ontario Lambert, EPSG: 5321) in R version 3.256.
Surface wind speeds and directions were modelled by the National Center for Environmental Prediction (NCEP) and obtained from the NOAA Operational Model Archive and Distribution System (NOMADS) (http://nomads.ncdc.noaa.gov/data/gfsanl/)57. Biases in the wind direction estimates were identified by comparing model outputs to empirical wind measured at the Churchill Airport, Manitoba (58.74°N, 94.07°W). Empirical wind data at six hour intervals were obtained from http://climate.weather.gc.ca/ (accessed on October 15, 2015).
NCEP generates gridded wind estimates at 6 hour intervals at 1° resolution (approximately 55 km longitude and 111 km latitude). To maximise the fit of wind data to movement data, only locations ≤4 hours apart were used. As the times and coordinates of both wind and movement data were not synchronized, wind data were spatially and temporally interpolated to match coordinates of bear locations. First, the wind was spatially interpolated to the location of the bear using inverse-distance weighting both before and after the time of a bear location58. Because wind estimates are both uniformly distributed in space (across a 1° grid) and have low resolution, the four wind estimates adjacent to a bear’s location were used. Second, the two spatial estimates were linearly interpolated to match the time of the location fix.
While on the sea ice, a portion of a bear’s absolute displacement is involuntary and driven by ice drift41,42. Thus, to study voluntary movement related to wind-associated foraging, the component ice drift was subtracted from the location data42. Ice drift data (Polar Pathfinder Daily 25 km EASE-Grid Sea Ice Motion Vectors) were acquired from the National Snow and Ice Data Center59. Ice drift was spatially interpolated using inverse distance weighting to match the bear locations58. The results of wind bias, and effect of removing ice drift are presented in the supplementary materials under ‘Wind model and ice drift bias.’
The data were filtered by four factors that may affect prevalence of cross-wind olfactory search: season, wind speed, bear speed, and presence of daylight. As habitat characteristics change over the year, and likely influence optimal foraging behaviour, we analyzed data separately by season: summer (on-land locations June 1 – October 31), autumn (on-land locations November 1–30), freeze-up (offshore locations November 1 – December 31), winter (offshore locations January 1 – June 30), and break-up (offshore locations July 1 – August 31)33. The overlap in the on-land and offshore seasons handles variation in sea ice formation across the Bay and between years, and individual variation in movement phenology.
As wind velocity plays a role in olfactory foraging efficiency, the data were subdivided into “slow” and “fast” wind categories. However, because we had no a priori threshold for wind speed at which behaviours change, we tested a moving threshold between 10.8 km/h and 54 km/h and presented the results in the supplementary material (Supplementary Tables S1 to S6). Although the whole range was tested, the presented data used thresholds that were representative of natural breaks in the moving threshold analysis (usually 36 km/h).Different bear speeds may reflect different behaviours (e.g., various olfactory search strategies, travel, rest, prey consumption). The 4-hour resolution of the collars likely includes periods of rest and movement. Additionally, the instantaneous bear velocity at each location may differ from the mean speed between successive locations because any deviations from the straight line path or variable velocities between the successive locations are not captured60. To limit analysis to potential olfactory search, we removed what we considered at-rest data (<0.01 km/h). To account for different behaviours and periods of rest data, the remaining movement data were divided into “slow” and “fast” bear speeds at thresholds between 0.5 km/h and 6 km/h between successive locations (Supplementary Tables S1 to S6). As with the wind speed threshold, the presented data used bear speed thresholds representative of natural breaks (usually 2 km/h). For each season, data were grouped into one of four categories: (1) slow wind and slow bears, (2) fast wind and slow bears, (3) slow wind and fast bears, and (4) fast wind and fast bears. Given our prediction of cross-wind search at moderate speeds, we did not expect predominant cross-wind orientation in categories (2) or (4) (due to fast winds). Intervals with slow bear speed were more likely to contain at-rest behaviour or still hunts, and so cross-wind orientation was not expected in categories (1) or (2) (due to slow bears). Thus, cross-wind orientation was predicted to be highest in category (3), slow winds and fast bears. For brevity, categories where predominant orientation relative to wind were similar were pooled and presented together. For example, “fast wind or slow bears” encompasses all data in categories (1), (2), and (4).
To test whether there was a circadian behavioural pattern, sunrise and sunset times were determined for each coordinate using the ‘sunriset’ function of ‘maptools’ package in R61. “Day” and “night” were defined by the sun being above or below the horizon, respectively, at each location.
Predominant bear direction relative to wind direction was assessed using χ2 tests. Data were binned into one of five directions: (1) tail winds (<25° between bear and wind bearings), (2) cross-tail winds (≥25° & <65°), (3) cross-wind (≥65° & <115°), (4) cross-head winds (≥115° & <155°), (5) and head winds (≥155° & ≤180°). Under the null hypothesis that bear direction is random with respect to wind direction, the expected ratio among the categories would be 5:8:10:8:5, respectively. These methods are adapted from Spear and Ainley, 199762, Weimerskirch et al.15, Paiva et al.63, and Zavalaga et al.64, which tested for anemotaxis during foraging using movement data relative to wind. Because of the moving thresholds of wind and bear speeds, each movement datum was analyzed within each wind/bear speed category. To control for multiple tests of each data point, a Bonferroni adjustment was made (statistical significance = 0.0006; based on 7 wind speed and 12 bear speed thresholds). If a set of data was statistically significant, adjusted standardized residuals were calculated to identify dominant orientation. All analyses were conducted both on the 4-hour collars and the 30-minute collars. To determine whether any patterns were artefacts of sampling rate, the 30-minute collar locations were sampled at a 4-hour interval by isolating all locations that were 4 hours apart. These results are presented in the supplementary material under ‘Sampling rate bias.’ To determine whether autocorrelation affected the observed patterns, we sampled the 4-hour collars by removing all steps <12 hours apart and ran the same χ2 tests. These results are presented under ‘Effect of autocorrelation.’ Temporal autocorrelation decay to random patterns as sampling time-lag is enforced65. As polar bear behaviours show diurnal patterns19 we assume that autocorrelation is negligible after 12 hours.
Means of unimodal distributions were calculated using the ‘mean.circular’ function from ‘circular’ package in R56. The two means of bimodal distributions were calculated using the ‘movMF’ package in R, which fits two von Mises-Fisher distributions using maximum likelihood66.
Results
Movement relative to geographic north
During summer, bears exhibited marginal bidirectionality with modes around −152° (SSW) and 15° (NNE) (Fig. 2a). During autumn, predominant movement was 0° (N), with northward movements nearly three times more frequent than eastward, westward, or southward movements (Fig. 2b). During freeze-up, predominant movement was 84° (E) (Fig. 2c). Winter and break-up movement exhibited bimodal distributions with modes around −33° (NNE) and 152° (SSE) (Fig. 2d and e).
Contribution of ice drift to displacement
During freeze-up and winter, when bears were moving slowly (<2 km/h) or when wind was fast (>36 km/h), bear orientation was unimodal with the mean displacement 20° relative to the wind bearing (Supplementary Fig. S1a). Movement with the component of ice drift removed was a mean −2° relative to the wind bearing (Supplementary Fig. S1b). We expected mean polar bear orientation to be symmetrical relative to the wind direction and not bias orientation toward the left or right of wind. As movement with ice drift removed deviated less from symmetry than without (Supplementary Fig. S1), all subsequent analyses were based on movement with ice drift removed.
Movement relative to wind
Predominant orientation of the bears was cross-wind during summer and autumn regardless of wind or bear speeds (Supplementary Tables S1 and S2). While winds were slow (<36 km/h) and polar bear speeds slow (<2 km/h), the two modes of summer movement were at 94° and −90° relative to the wind bearing (Fig. 3a). During autumn, the two modes were at 79° and −85° relative to wind, with the latter being more frequent (Fig. 3b). Although the orientation was statistically significant (summer, χ2 = 42, df = 4, P < 0.0001; autumn, χ2 = 68, df = 4, P<0.0001), bear orientation was more strongly associated with angle to north (angular dispersion = 0.24) than with wind bearing (angular dispersion = 0.12) (Fig. 2a and b vs. Fig. 3; Kruskal-Wallis χ2 = 48, df = 1, P < 0.0001, Wallraff test of angular dispersion).
During freeze-up, the predominant orientation of the bears relative to wind was linked to both polar bear and wind speeds. Slower bear movements (<2 km/h) or movements while wind was fast ( > 21.6 km/h) were predominantly tail wind (Supplementary Table S3; Fig. 4a, χ2 = 5002, df = 4, P < 0.0001). Orientation was more strongly associated with angle to wind (angular dispersion = 0.46) than with north (angular dispersion = 0.25) (Fig. 2c vs. Fig. 4; Kruskal-Wallis χ2 = 681, df = 1, P < 0.0001, Wallraff test of angular dispersion). Fast polar bear movements (>2 km/h) while wind was slow (<21.6 km/h) were predominantly cross-wind (Supplementary Table S3; Fig. 4b), with modes at 90° and −100° relative to the wind (Supplementary Table S3; Fig. 4b; χ2 = 77, df = 4, P < 0.0001), however, only 8% (n = 882) of the freeze-up data fell into this category.
Frequency of polar bear orientation relative to wind while (a) polar bear speed was <2 km/h or wind speed was >21.6 km/h and (b) polar bear speed was >2 km/h and wind speed was <21.6 km/h. Curves represent probability density functions based on maximum likelihood of a single (for a) and a mixture of two (for b) von Mises-Fisher distributions.
During winter, slower bear movements (<2 km/h) or movements while wind was fast ( > 36 km/h) were predominantly tail wind (Supplementary Table S4; Fig. 5a; mode = −1 °, χ2 = 8520, df = 4, P < 0.0001). Fast polar bear movements (>2 km/h) while wind was slow (<36 km/h) were predominantly cross-wind (Supplementary Table S4; Fig. 5b; mode1 = 81°, mode2 = −102°, χ2 = 275, df = 4, P < 0.0001). Dividing the fast bear and slow wind data into day and night revealed a circadian pattern, with more cross-wind movement at night than during the day (Fig. 5b). As with the 4-hour collars, 30-minute collars exhibited predominantly downwind movement during slow bear movement or under fast winds (Supplementary Table S5; Fig. 5c; mode = −3 °, χ2 = 649, df = 4, P < 0.0001), whereas fast bear movements under slow winds were predominantly cross-wind (Supplementary Table S5; Fig. 5d; mode1 = 90°, mode2 = −109°, χ2 = 113, df = 4, P < 0.0001). For the 30-minute data, 26% of winter data fell into the ‘slow wind and fast bear’ category, compared to 10% of the 4-hour winter data.
Frequency of polar bear orientation relative to wind while polar bear speed was <2 km/h or wind speed was >36 km/h (a and c - representing two collar types, see below), and while polar bear speed was >2 km/h and wind speed was <36 km/h (b and d - representing two collar types, see below). (a and b) represent collars that had 4-hour fix intervals while (c and d) represent collars that had 30-minute fix intervals. (b) is subset into day (light grey) and night (dark grey). Curves represent probability density functions based on maximum likelihood of a single (for a and c) and a mixture of two (for b and d) von Mises-Fisher distributions.
During ice break-up, polar bear movements were predominantly cross-tail wind with a unimodal orientation of 34° relative to the wind, regardless of collar location frequency or bear or wind speeds (Supplementary Table S6; Fig. 6; χ2 = 89, df = 4, P < 0.0001). All orientation patterns described above are consistent when subsampled at 12-hour intervals to remove autocorrelation (Supplementary Table S7).
Discussion
We observed polar bear movement patterns that were associated with season, presence of daylight, wind speed, and bear speed. Seasons vary in food distribution and habitat conditions34,36,67. As food abundance and distribution change and as energetic cost of foraging change, different foraging behaviours may be optimal32,38,68. Within any season, wind speed can influence the effectiveness of olfactory search. Higher wind speeds increase turbulence and affect odour filament distribution and may be unfavourable for olfaction13,46,47. In addition, bear speed might reflect different behaviours, only some of which are cross-wind search19. Other behaviours such as travel between patches, migration, rest, or visual search likely exhibit different relationships with wind than cross-wind search.
Distinguishing between behaviours is complicated by the delineation of biologically meaningful seasons and by the resolution and accuracy of the wind estimation, ice drift estimation, and GPS tracking data. Although the modelled wind direction was a mean 10° left of wind measured at Churchill Airport (Supplementary Fig. S2), it falls within the bin sizes used in the χ2 tests (±25° or ±20°, depending on orientation), and would not cause misclassification of the predominant direction. The 4-hour resolution of the location data can only capture sustained movements, masking short-term responses to wind, such as upwind localization of a detected odour. Despite the inherent challenges and limitations of studying animals with vast and remote ranges, we observed several wind- and season-associated behaviours.
In Hudson Bay, terrestrial foraging by polar bears is limited to less energetically dense foods (e.g., berries, and seaweed)34,35,36,37, and the bears minimize unnecessary movement to conserve energy32,38,39. We found a weak (though significant) association between bear movement and wind during summer, suggesting that cross-wind search is either reduced or absent during this season (Fig. 3a). We found a similar bimodal distribution in movement relative to north, with bears tending to move north or south (Fig. 2a). Any movement during the summer would be confined by the shoreline, which extends north-south in western Hudson Bay (Fig. 1). Because of the predominantly northwesterly winds (Supplementary Fig. S3), random movement confined by the shoreline would tend to also be cross-wind. Thus, the cross-wind movement we observed during summer may be an artefact of the landscape rather than a response to wind.
Freeze-up begins in northwest Hudson Bay69 and, by moving northwards during the months leading up to freeze-up, bears are able to return to the sea ice sooner. The northward movement we observed during autumn (Fig. 3b) may be an example of polar bears’ migratory behaviour associated with freeze-up32.
Polar bears traveled predominantly downwind during freeze-up (Fig. 4a), which leads them east towards the centre of the Bay (Fig. 2c). In addition to following the southeastward advancing sea ice, we suggest the movement may be partly guided by wind. Because wind direction was variable (Supplementary Fig. S3), movement guided solely by celestial or global cues (such as solar position or global magnetisms)41 would have a stronger association relative to north than relative to wind, which was not the case (Fig. 2c vs Fig. 4a). As intraspecific competition affects distribution of female polar bears with cubs70, the focus of such bears during freeze-up may be to disperse throughout the Bay and away from conspecifics. The pattern of dispersal during freeze-up may be sex-specific as females avoid males due to the threat of infanticide33. However, we cannot draw conclusions regarding male movement, because only females with young were collared.
We observed cross-wind movement during freeze-up, however, only at low wind and high bear speeds (Fig. 4b). Because predominant cross-wind movement during freeze-up was found only at lower wind speeds (<21.6 km/h) compared to winter (<36 km/h), it suggests that some foraging during freeze-up may occur.
During winter, at high wind speeds or when polar bears were moving slowly movement was predominantly downwind (Fig. 5a) and was not predicted. Explanations for the downwind movement include that it may represent a default orientation that generally leads bears southeast and further into the Bay. Second, it may be a thermoregulatory response to high wind speeds; moving downwind minimises the surface area of a bear exposed to wind and shields the face. Thermoregulatory downwind orientation has been modelled and observed for several taxa71,72. Third, if wind direction fluctuates more than 30° from the mean, then upwind or downwind movement provide more information about the environment than cross-wind movement, a phenomenon described as the geometric pattern of scent dispersion8,13. However, the geometric pattern of scent dispersion alone cannot account for the low frequency of upwind movement observed. Finally, behaviours apart from cross-wind search, such as travel, still-hunting, movement following habitat features, or other olfactory search strategies may tend to be downwind. Non-olfactory behaviours occupy around 60% of polar bear time budgets19,25.
During winter, at low wind speeds and while polar bear speeds were high, movement was predominantly cross-wind (Fig. 5b) and matched our predicted movement for cross-wind olfactory search. If the cross-wind movement during freeze-up and winter is reflective of cross-wind olfactory search, the greater frequency at lower wind speeds aligns with findings that polar bear hunting success increases with decreasing wind speed73. Cross-wind movement was also more common at night than during the day (Fig. 5b), supporting our hypothesis that movement is primarily guided by olfaction during periods of darkness, while movement during the day may rely, in part, on visual cues. We observed cross-wind movement more frequently among bears wearing collars that had 30-minute fix rates than those wearing collars with 4-hour fix rates (Fig. 5b vs. d). Additionally, the proportion of 30-minute data in the slow wind and fast bear category was 2.6 times greater than among 4-hour collars, suggesting that directionalities observed among the lower resolution collars underestimated the proportion of cross-wind movement. Additionally, the 30-minute data that was subsampled at 4-hour intervals revealed the same patterns as non-subset 30-minute data and 4-hour data, where movement was downwind while winds were fast or bears were slow, and movement was cross-wind while winds were slow and bears were fast (Supplementary Fig. S5), suggesting that the observed patterns were not an artefact of sampling rate.
An alternative explanation for cross-wind movement is that it is a response to environmental features that are associated with the predominant winds, such as pressure ridges74. However, environmental features cannot account for the association between bear orientation and wind speed or presence of daylight, as was exhibited during freeze-up and winter. Specifically, the presence of pressure ridges is independent of wind speed and presence of daylight, while cross-wind movement was dependent on these factors. Additionally, because of the counter-clockwise gyre in Hudson Bay and changing winds, pressure ridges in the Bay are not uniformly cross-wind.
During break-up, mean polar bear movement was 34° relative to the wind (Fig. 6). With the predominant northwesterly winds, this would take the bears southeast towards shore and following the retreating ice. The movement relative to north shows a large component of northwestward movement (Fig. 2e), which may reflect bears compensating for ice drift41,42 before returning to land as hunting conditions deteriorate. As the season progresses and sea ice melts, polar bears may spend increasingly more time swimming, during which collars cannot transmit locations75,76. As such, limiting analysis to only 4-hour collars does capture the complete range of behaviours, especially during break-up.
We predicted that polar bears employ cross-wind olfactory search and predicted that this would occur more frequently during winter, when they enter hyperphagia54, under moderate wind speeds, when conditions are conducive for olfaction 73,77,78, at higher bear speeds, when they are more likely engaged in active search, and at night, when olfaction may be more effective than visual search45. The observed cross-wind movement during winter generally supports our hypotheses and predictions. Olfactory foraging may vary across populations due to patterns of sea ice distribution and raises additional research questions. Does maintenance of relative position on drifting ice41,42 come at the expense of prolonged olfactory search? Polar bears on more stable ice may be less active than bears on drifting ice38 - what is the role of olfaction in more stable habitats? Arctic wind speeds are projected to increase due to climate change79 and could impede polar bear hunting success73. Further studies using higher temporal resolution location data, in combination with direct observation of hunting bears, would further our understanding of olfactory predation. Additionally, given its influence on behaviour, wind could be incorporated as a characteristic in habitat selection modelling of olfactory predators, as the quality of a habitat may be dependent on windscapes. In practice, windscapes could be used as modifiers to the available habitat (e.g., fast winds invoke downwind movement, while cross-wind movement would be favoured under moderate winds). To our knowledge, this is the first such evidence of cross-wind orientation for olfactory search for any wild, non-avian carnivore. The methods presented here are widely applicable and can provide insight on olfactory search among predators across taxa (e.g., canids, felids, and mustelids) and on prey using olfaction to avoid predators.
Additional Information
How to cite this article: Togunov, R. R. et al. Windscapes and olfactory foraging in a large carnivore. Sci. Rep. 7, 46332; doi: 10.1038/srep46332 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
31 May 2018
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
31 May 2018
Scientific Reports 7: Article number: 46332; published online: 12 April 2017; updated: 31 May 2018 This Article contains errors where the ice drift vectors data were incorrectly labelled. Ice drift vectors data is stored as two orthogonal vectors, the horizontal and vertical components, ‘u’ and ‘v’.In the EASE-grid projection used for the ice drift, the cardinal directions of ‘u’ and ‘v’ depend on the longitude.
References
Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: a selective review of theory and tests. Q. Rev. Biol. 52, 137–154 (1977).
Higginson, A. D. & Ruxton, G. D. Foraging mode switching: the importance of prey distribution and foraging currency. Anim. Behav. 105, 121–137 (2015).
Morse, D. H. & Fritz, R. S. Experimental and observational studies of patch choice at different scales by the crab spider Misumena vatia . Ecol. Soc. Am. 63, 172–182 (1982).
Charnov, E. L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136 (1976).
Stein, R. A. Selective predation, optimal foraging, and the predator-prey interaction between fish and crayfish. Ecol. Soc. Am. 58, 1237–1253 (1977).
Sims, D. W. et al. Scaling laws of marine predator search behaviour. Nature 451, 1098–1102 (2008).
Gittleman, J. L. Carnivore olfactory bulb size: allometry, phylogeny and ecology. J. Zool. 225, 253–272 (1991).
Bau, J. & Cardé, R. T. Modeling optimal strategies for finding a resource-linked, windborne odor plume: Theories, robotics, and biomimetic lessons from flying insects. Integr. Comp. Biol. 55, 461–477 (2015).
Murlis, J., Willis, M. A. & Cardé, R. T. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol. Entomol. 25, 211–222 (2000).
Farrell, J. A., Murlis, J., Long, X., Li, W. & Cardé, R. T. Filament-based atmospheric dispersion model to achieve short time-scale structure of odor plumes. Environ. fluid Mech. 2, 143–169 (2002).
Cardé, R. T. & Willis, M. A. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 34, 854–866 (2008).
Dusenbery, D. B. Optimal search direction for an animal flying or swimming in a wind or current. J. Chem. Ecol. 15, 2511–2519 (1989).
Sabelis, M. W. & Schippers, P. Variable wind directions and anemotactic strategies of searching for an odour plume. Oecologia 63, 225–228 (1984).
Kennedy, J. S. & Marsh, D. Pheromone-regulated anemotaxis in flying moths. Science 184, 999–1001 (1974).
Weimerskirch, H., Le Corre, M., Ropert-Coudert, Y., Kato, A. & Marsac, F. The three-dimensional flight of red-footed boobies: adaptations to foraging in a tropical environment? Proc. R. Soc. 272, 53–61 (2005).
Nevitt, G. A., Losekoot, M. & Weimerskirch, H. Evidence for olfactory search in wandering albatross, Diomedea exulans . P. Natl. Acad. Sci. USA 105, 4576–4581 (2008).
Hirsch, B. T. Tradeoff between travel speed and olfactory food detection in ring-tailed coatis (Nasua nasua). Ethology 116, 671–679 (2010).
Derocher, A. E., Lunn, N. J. & Stirling, I. Polar bears in a warming climate. Integr. Comp. Biol. 44, 163–176 (2004).
Stirling, I. Midsummer observations on the behavior of wild polar bears (Ursus maritimus). Can. J. Zool. 52, 1191–1198 (1974).
Smith, T. G. Polar bear predation of ringed and bearded seals in the land-fast sea ice habitat. Can. J. Zool. 58, 2201–2209 (1980).
Pilfold, N. W., Derocher, A. E., Stirling, I., Richardson, E. & Andriashek, D. Age and sex composition of seals killed by polar bears in the Eastern Beaufort sea. PLoS One 7, e41429 (2012).
Chambellant, M., Lunn, N. J. & Ferguson, S. H. Temporal variation in distribution and density of ice-obligated seals in western Hudson Bay, Canada. Polar Biol. 35, 1105–1117 (2012).
Smith, T. G. & Stirling, I. The breeding habitat of the ringed seal (Phoca hispida). The birth lair and associated structures. Can. J. Zool. 53, 1297–1305 (1975).
Strub-Klein, L. & Sudom, D. A comprehensive analysis of the morphology of first-year sea ice ridges. Cold Reg. Sci. Technol. 82, 94–109 (2012).
Stirling, I. & Latour, P. B. Comparative hunting abilities of polar bear cubs of different ages. Can. J. Zool. 56, 1768–1772 (1978).
Tucker, M. A., Ord, T. J. & Rogers, T. L. Evolutionary predictors of mammalian home range size: body mass, diet and the environment. Glob. Ecol. Biogeogr. 23, 1105–1114 (2014).
Lai, S., Bêty, J. & Berteaux, D. Spatio–temporal hotspots of satellite–tracked arctic foxes reveal a large detection range in a mammalian predator. Mov. Ecol. 3, 1–10 (2015).
Novak, M. Wild furbearer management and consevration in North America(ed. Novak, M. et al.) 474–485 (Ontario Trappers Association, 1987).
Kingsley, M. C. S. & Stirling, I. Haul-out behaviour of ringed and bearded seals in relation to defence against surface predators. Can. J. Zool. 69, 1857–1861 (1991).
Hardy, M. H., Roff, E., Smith, T. G. & Ryg, M. Facial skin glands of ringed and grey seals, and their possible function as odoriferous organs. Canadian Journal of Zoology 69, 189–200 (1991).
Owen, M. A. et al. An experimental investigation of chemical communication in the polar bear. J. Zool. 295, 36–43 (2015).
Derocher, A. E. & Stirling, I. Distribution of polar bears (Ursus maritimus) during the ice-free period in western Hudson Bay. Can. J. Zool. 68, 1395–1403 (1990).
McCall, A. G., Derocher, A. E. & Lunn, N. J. Home range distribution of polar bears in western Hudson Bay. Polar Biol. 38, 343–355 (2014).
Derocher, A. E., Andriashek, D. & Stirling, I. Terrestrial foraging by polar bears during the ice-free period in western Hudson Bay. Arctic 46, 251–254 (1993).
Russell, R. H. The Food Habits of Polar Bears of James Bay and Southwest Hudson Bay in Summer and Autumn. Arctic 28, 117–129 (1975).
Rode, K. D., Robbins, C. T., Nelson, L. & Amstrup, S. C. Can polar bears use terrestrial foods to offset lost ice-based hunting opportunities? Front. Ecol. Environ. 13, 138–145 (2015).
Pilfold, N. W. et al. Mass loss rates of fasting polar bears. Physiol. Biochem. Zool. 89, 377–388 (2016).
Ferguson, S. H., Taylor, M. K., Born, E. W., Rosing-Asvid, A. & Messier, F. Activity and movement patterns of polar bears inhabiting consolidated versus active pack ice. Arctic 54, 49–54 (2001).
Rozhnov, V. V. et al. Movements of polar bear females (Ursus maritimus) during an ice-free period in the fall of 2011 on Alexandra Land Island (Franz Josef Land Archipelago) using satellite telemetry. Biol. Bull. 42, 728–741 (2015).
Stirling, I. & McEwan, E. H. The caloric value of whole ringed seals (Phoca hispida) in relation to polar bear (Ursus maritimus) ecology and hunting behavior. Can. J. Zool. 53, 1021–1027 (1975).
Mauritzen, M., Derocher, A. E., Pavlova, O. & Wiig, Ø. Female polar bears, Ursus maritimus, on the Barents Sea drift ice: walking the treadmill. Anim. Behav. 66, 107–113 (2003).
Auger-Méthé, M., Lewis, M. A. & Derocher, A. E. Home ranges in moving habitats: Polar bears and sea ice. Ecography 39, 26–35 (2016).
Cherry, S. G., Derocher, A. E. & Lunn, N. J. Habitat-mediated timing of migration in polar bears: an individual perspective Manuscript. Ecol. Evol. 6, 5032–5042 (2016).
Stirling, I., Lunn, N. J. & Iacozza, J. Long-term trends in the population ecology of polar bears in western Hudson Bay in relation to climatic change. Arctic 52, 294–306 (1999).
Balkenius, A., Rosén, W. & Kelber, A. The relative importance of olfaction and vision in a diurnal and a nocturnal hawkmoth. J. Comp. Physiol. A 192, 431–437 (2006).
Cablk, M. E., Sagebiel, J. C., Heaton, J. S. & Valentin, C. Olfaction-based detection distance: A quantitative analysis of how far away dogs recognize tortoise odor and follow it to source. Sensors 8, 2208–2222 (2008).
Brady, J., Griffiths, N. & Paynter, Q. Wind speed effects on odour source location by tsetse flies (Glossina). Physiol. Entomol. 20, 293–302 (1995).
Prinsenberg, S. J. Freshwater contents and heat budgets of James Bay and Hudson Bay. Cont. Shelf Res. 3, 191–200 (1984).
Saucier, F. J. et al. Modelling the sea ice-ocean seasonal cycle in Hudson Bay, Foxe Basin and Hudson Strait, Canada. Clim. Dyn. 23, 303–326 (2004).
Danielson, E. W. Hudson Bay ice conditions. Arctic 24, 90–107 (1971).
Gough, W. A., Cornwell, A. R. & Tsuji, L. J. S. Trends in seasonal sea ice duration in southwestern Hudson Bay. Arctic 57, 299–305 (2004).
Etkin, D. A. Break-up in Hudson Bay: its sensitivity to air temperatures and implications for climate warming. Climatol. Bull. 25, 21–34 (1991).
Regehr, E. V., Lunn, N. J., Amstrup, S. C. & Stirling, I. Effects of earlier sea ice breakup on survival and population size of polar bears in western Hudson Bay. J. Wildl. Manage. 71, 2673–2683 (2007).
Ramsay, M. A. & Stirling, I. Reproductive biology and ecology of female polar bears (Ursus maritimus). J. Zool. 214, 601–634 (1988).
Stirling, I., Spencer, C. & Andriashek, D. Immobilization of polar bears (Ursus maritimus) with Telazol ® in the Canadian Arctic. J. Wildl. Dis. 25, 159–168 (1989).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org (2016).
Bowman, D. C. & Lees, J. M. Near real time weather and ocean model data access with rNOMADS. Comput. Geosci. 78, 88–95 (2015).
Li, J. & Heap, A. D. A review of comparative studies of spatial interpolation methods in environmental sciences: performance and impact factors. Ecol. Inform. 6, 228–241 (2011).
Fowler, C., Maslanik, J., Emery, W. & Tschudi, M. Polar Pathfinder daily 25 km EASE-Grid sea ice motion vectors. Version 2. [January 2004 - December 2012]. Boulder, Colorado USA National Snow and Ice Data Center., distributed in netCDF format by the Integrated Climate Data Center (ICDC, http://icdc.zmaw). (University of Hamburg, 2013).
Rowcliffe, J. M. & Carbone, C. Bias in estimating animal travel distance: the effect of sampling frequency. Methods Ecol. Evol. 3, 653–662 (2012).
Lewin-Koh, N. & Bivand, R. maptools: tools for reading and handling spatial objects. R package version 0.8–27 (2013).
Spear, L. B. & Ainley, D. G. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis. 139, 221–233 (1997).
Paiva, V. H. et al. Flight dynamics of Cory’s shearwater foraging in a coastal environment. Zoology 113, 47–56 (2010).
Zavalaga, C. B., Halls, J. & Dell’Omo, G. Marine habitat use of Peruvian boobies: a geographic and oceanographic comparison between inshore and offshore islands. ICES J. Mar. Sci. 67, 940–951 (2010).
Boyce, M. S. et al. Temporal autocorrelation functions for movement rates from global positioning system radiotelemetry data. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 365, 2213–2219 (2010).
Hornik, K. & Grün, B. movMF: an R package for fitting mixtures of von Mises-Fisher distributions. J. Stat. Softw. 58, 1–31 (2014).
Gormezano, L. J. & Rockwell, R. F. What to eat now? Shifts in polar bear diet during the ice-free season in western Hudson Bay. Ecol. Evol. 3, 3509–3523 (2013).
Sims, D. W., Humphries, N. E., Bradford, R. W. & Bruce, B. D. Lévy flight and Brownian search patterns of a free-ranging predator reflect different prey field characteristics. J. Anim. Ecol. 81, 432–442 (2012).
Gagnon, A. S. & Gough, W. A. Trends in the dates of ice freeze-up and breakup over Hudson Bay, Canada. Arctic 58, 370–382 (2005).
Pilfold, N. W., Derocher, A. E. & Richardson, E. Influence of intraspecific competition on the distribution of a wide-ranging, non-territorial carnivore. Glob. Ecol. Biogeogr. 23, 425–435 (2014).
DeMatteo, K. E. & Harlow, H. J. Thermoregulatory responses of the North American porcupine (Erethizon dorsatum bruneri) to decreasing ambient temperature and increasing wind speed. Comp. Biochem. Physiol. 116, 339–346 (1997).
Timisjärvi, J., Nieminen, M. & Sippola, A. L. The structure and insulation properties of the reindeer fur. Comp. Biochem. Physiol. 79, 601–609 (1984).
Pilfold, N. W., Derocher, A. E., Stirling, I. & Richardson, E. Multi-temporal factors influence predation for polar bears in a changing climate. Oikos 124, 1098–1107 (2015).
Parmerter, R. R. & Coon, M. D. Model of pressure ridge formation in sea ice. J. Geophys. Res. 77, 6565–6575 (1972).
Pagano, A. M., Durner, G., Amstrup, S. C., Simac, K. S. & York, G. S. Long-distance swimming by polar bears (Ursus maritimus) of the southern Beaufort Sea during years of extensive open water. Can. J. Zool. 90, 663–676 (2012).
Pilfold, N. W., McCall, A. G., Derocher, A. E., Lunn, N. J. & Richardson, E. Migratory response of polar bears to sea ice loss: to swim or not to swim. Ecography 39, 1–11 (2016).
Van Eerden, M. R. & Voslamber, B. Mass fishing by cormorants Phalacrocorax carbo sinensis at Lake Ijsselmeer, the Netherlands: a recent and successful adaptation to a turbid environment. Ardea 83, 199–212 (1995).
Conover, M. R. Predator-Prey Dynamics: The Role of Olfaction.(CRC Press, 2007).
McInnes, K. L., Erwin, T. A. & Bathols, J. M. Global Climate Model projected changes in 10 m wind speed and direction due to anthropogenic climate change. Atmos. Sci. Lett. 12, 325–333 (2011).
QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation ProjectAvailable at: http://www.qgis.org/en/site/ (2016).
Acknowledgements
We thank the Churchill Northern Studies Centre for support in the field. Funding was provided by ArcticNet, Canadian Association of Zoos and Aquariums, Canadian Wildlife Federation, Care for the Wild International, Earth Rangers Foundation, Environment and Climate Change Canada, EnviroNorth, Hauser Bears, the Isdell Family Foundation, Manitoba Sustainable Development, Natural Sciences and Engineering Research Council of Canada, Parks Canada, Polar Bears International, Sigmund Soudack & Associates Inc., Quark Expeditions, the University of Alberta, Wildlife Media Inc., and World Wildlife Fund (Canada).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research questions. R.R.T. conducted the analyses and drafted the manuscript; all authors contributed substantially to revisions. N.J.L. and A.E.D. were responsible for GPS collar deployment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Togunov, R., Derocher, A. & Lunn, N. Windscapes and olfactory foraging in a large carnivore. Sci Rep 7, 46332 (2017). https://doi.org/10.1038/srep46332
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep46332
This article is cited by
-
Olfactory system structure and function in newly hatched and adult locusts
Scientific Reports (2024)
-
Behavioral-dependent recursive movements and implications for resource selection
Scientific Reports (2023)
-
Drivers of polar bear behavior and the possible effects of prey availability on foraging strategy
Movement Ecology (2022)
-
The overlap of sympatric sun bears and Asiatic black bears in space and time
Mammalian Biology (2022)
-
Behavioural adjustments of predators and prey to wind speed in the boreal forest
Oecologia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.