Abstract
Camptothecin (CPT) and X-ray (XR) generate double-strand breaks (DSB) that can be processed by homologous or nonhomologous recombination. We studied the participation of proteins involved in recombination pathways and cell cycle control in the signal transduction between DNA damage and NF-κB. Cells harbouring mutated NBS, hMRE11, BRCA1 or MLH1 were analysed. NBS- and hMRE11-deficient cells present a classical kinetic of NF-κB induction after camptothecin treatment. When DSB are generated by XR, NBS-deficient cells exhibit a delayed and strongly reduced level of NF-κB induction, whereas the hMRE11 mutated cells do not induce NF-κB at all. This indicates an important role of the hMRE11/hRAD50/NBS complex in the signal transduction initiated by XR. In HCC1937 cells that express a truncated version of BRCA1, XR induces a very rapid and transient NF-κB activation, whereas CPT leads to a delayed activation suggesting that BRCA1 modulates the transduction pathways in different manners after these two stresses. Finally, we found that a proficient MMR pathway is essential to the NF-κB activation after both CPT and XR. These results indicate that DSB originating from XR or CPT do not induce NF-κB in a unique way. MMR participates in both cascades, whereas the hMRE11/hRAD50/NBS trimer is specifically involved in the response elicited by XR.
Similar content being viewed by others
Introduction
NF-κB is a transcription factor activated in response to multiple agents such as TNFα, IL1β, LPS, oxidant and DNA-damaging drugs (Bharti and Aggarwal, 2002; Gosh and Karin, 2002; Karin et al., 2002). In unstimulated cells, NF-κB is maintained inactive in the cytoplasm by a family of inhibitors, the IκBs. Upon stimulation, the inhibitor is generally degraded and NF-κB is then free to move into the nucleus where it can activate its target genes. NF-κB activation by camptothecin (CPT) and X-ray (XR) has been largely documented, and DNA damage has been shown to be the triggering event (Piret and Piette, 1996; Huang et al., 2000; Habraken et al., 2001; Li et al., 2001). Several of the last steps immediately preceding the transfer of NF-κB into the nucleus were identified: (i) activation of the IκBα kinase (IKK) complex, (ii) IκBα phosphorylation with its subsequent ubiquitination and, (iii) degradation of IκBα by the 26S proteasome (Huang et al., 2000; Habraken et al., 2001). One of the steps upstream of the activation of the IKK relies on RIP, since RIP−/− cells fail to activate NF-κB after both CTP and IR treatments (Hur et al., 2003). However, there are still large gaps in the knowledge of events taking place between the initiating DNA damage and the activation of the IKK complex in the cytoplasm.
In this work, we studied the nuclear events that are involved in NF-κB activation following double-strand break (DSB) induction by CPT and XR. We focused our attention on the participation of several proteins involved in homologous recombination and/or nonhomologous end joining (HR and NHEJ) as well as in the control of cell cycle checkpoints. CPT and its soluble analogs (irinotecan and topotecan) have antitumour activities (Pommier et al., 1998). Irinotecan and topotecan are used in the treatment of colon cancer and ovarian cancer, respectively. They act by binding to the DNA-topoisomerase I cleavage complex and prevent the religation step. During DNA replication, the replication fork collides with the ‘trapped’ topo-I-DNA complexes and leads to the formation of DSB. Topo-I is the only cellular target of CPT, therefore all breaks are identical and occur during the S-phase of the cell cycle (Avemann et al., 1998; Pommier et al., 1998). The main cytotoxic DNA lesions generated by XR are also DSB, but the nature of the DNA breakage extremities is multiple and not associated with the collapse of the replication fork (Friedberg et al., 1995).
Firstly, we tested the potential involvement of the hMRE11/hRAD50/NBS (M/R/N) heterotrimer in this signal transduction. Ataxia Telangiectasia (AT), Ataxia Telangiectasia Like Disease (ATLD) and Nijmegen Breakage Syndrome (NBS) are the three rare human autosomal diseases exhibiting similar clinical features such as hypersensitivity to ionizing radiation (IR), immunodeficiency and increased predisposition to develop cancer (Shiloh, 1997; Varon et al., 1998; Stewart et al., 1999; Petrini, 2000). At the cellular level AT, ATLD and NBS cells present chromosome instability, hypersensitivity to genotoxic stress and cell cycle checkpoint defects. ATM is the only nuclear protein shown to be an intermediate in NF-κB activation by DSB, since NF-κB activation by CPT or IR is reduced in intensity and duration in AT-deficient cell lines (Piret et al., 1999; Li et al., 2001). NBS associates with hMRE11/hRAD50 to form the M/R/N complex. This trimer plays a vital role in many cellular processes involving DNA extremities, such as (i) DSB repair by both HR and NHEJ, (ii) sensing and signalling DNA breaks to the cell cycle regulatory apparatus and (iii) acting at the level of the S-phase checkpoint (Thompson and Schild, 2002). The observation that both NBS and hMRE11 are phosphorylated by ATM upon IR (Gatei et al., 2000; Lim et al., 2000; D'amours and Jackson, 2002) prompted us to investigate the role of these two proteins in the signal transduction leading to NF-κB activation in response to DSB.
Secondly, we examined the influence of a BRCA1 truncation in this signal cascade. BRCA1 is a tumour suppressor gene that is mutated in approximately 40–50% of the inherited breast cancer. BRCA1 protein has numerous roles and ensures, at least in part, a genomic stability through a functional role in DNA damage repair. BRCA1-deficient cells are hypersensitive to IR and exhibit a slower rate and less extensive DSB repair (Scully et al., 1999). BRCA1 is involved in both NHEJ and HR (Venkitaraman, 2001; Zhong et al., 2002) and can bind directly to DNA (Paull et al., 2001) and to the M/R/N trimer (Zhong et al., 1999). After IR, BRCA1 is phosphorylated by ATM (Cortez et al., 1999) and then controls cell cycle checkpoints in the S-phase and at the G2/M border (Xu et al., 2002).
Finally, as it was recently shown that MMR-deficient cell lines are hypersensitive to CPT (Jacob et al., 2001; Pichierri et al., 2001), we tested the involvement of MLH1 in the signal transduction initiated by CPT and XR. MLH1 plays an essential role in the early steps of the MMR pathway. MMR inactivation is associated with hereditary nonpolyposis colorectal cancer (HNPCC), a syndrome characterized by an early incidence of colon, endometrium and ovary cancers. MMR pathway is involved not only in the removal of replicative DNA polymerase errors, but some of its components are also implicated in HR and cell cycle control both at the G2/M border and at an S-phase checkpoint (Harfe and Jinks-Robertson, 2000; Pichierri et al., 2001; Jacob and Praz, 2002; Brown et al., 2003). These second and third characteristics of MMR could be important for the repair of DSB generated either by CPT or IR.
Here, we report that the M/R/N trimer participates in NF-κB activation when DNA damage is generated by XR, but not by CPT. We also present evidences that wild-type MLH1 is essential for the signal transduction after both types of damage suggesting that the MMR pathway is involved in these cascades. BRCA1 protein is more likely to have a modulator function as the kinetics of NF-κB activation are either delayed or accelerated in cells carrying mutated BRCA1, depending on the nature of the lesions. Taken together, these data infer that at least two different pathways transduce the signal leading to NF-κB activation following DNA damage.
Results
NBS participates in the cellular responses initiated by CPT and XR
NBS-ILB1 cell line (homozygous for the deletion 657del5) and its corrected isogenic counterpart (NBS-ILB1-6, with full-length NBS reintroduced by a retrovirus) were used in this study (Kraakman-Van Der Zwet et al., 1999; Cerosaletti et al., 2000). Both cell lines have an identical doubling time (data not shown). The sensitivity of NBS-deficient and corrected cells towards CPT and XR was assessed by clonogenic assay. In Figure 1a (left panel), the cells were exposed for 24 h to increasing doses of CPT and washed with fresh media and then allowed to grow for 14 days. At that time, the colonies formed were counted. It can be seen that the expression of full-length NBS decreases the sensitivity towards CPT. The shoulder in the survival curve observed at the lowest concentration (5 nM CPT) indicates that repair is taking place in the corrected cell line. We could not observe any differences in the survival rate of the two cell lines using an assay based on the measurement of the mitochondrial respiration after 24 or 48 h (data not shown).
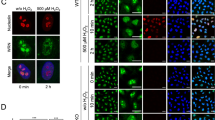
NBS participates to the cellular response elicited by XR and CPT. (a) Survival of NBS-ILB1 and NBS-ILB1-6 cells following irradiation or CPT-addition. NBS-ILB1 and NBS-ILB1-6 cells were plated at clonal concentration and incubated 24 h with increasing doses of CPT. After an extensive washing, the cells were grown for 14 days in complete media (left panel). Exponentially growing NBS-ILB1 and NBS-ILB1-6 cells were XR-treated with 0, 2, 4 or 6 Gy. Immediately after irradiation, the cells were plated at different densities and grown for 14 days (right panel). At that time, the colonies present on each plate were counted and expressed as a percentage of the untreated cells. Each point represents the mean value of two independent experiments with triplicates (±s.d.). NBS-ILB1-6 (filled square), NBS-ILB1 (open diamond). (b) Phosphorylation of NBS in HeLa cells after both CPT and XR treatment. The presence of phosphorylated NBS (P-NBS) in 10 μg of nuclear extracts of HeLa cells treated with 10 μ M CPT for ¼, ½, 1 and 2 h or irradiated with 20 Gy and then subsequently incubated for 2 h was detected by Western blotting. The position of NBS and P-NBS, which migrates slightly more slowly, are both indicated. (c) Nuclear foci formation after CPT addition. The subcellular distribution of NBS in CPT-treated HeLa cells was determined by immunofluorescence. Control and CPT-treated HeLa cells (10 μ M) for 30, 90 min or 24 h (with and without removal of CPT) were fixed and stained with an antibody specific to NBS (magnification × 1000, the cells were counterstained with Evans blue). A secondary antibody coupled to FITC was used to visualize the foci under fluorescence
In Figure 1a (right panel), NBS-ILB1 and NBS1-ILB1-6 cells were exposed to increasing doses of XR, immediately plated thereafter at different dilution and allowed to grow. The colonies formed were counted after 14 days. As already reported for γ-ray (Cerosaletti et al., 2000), we can observe that the corrected cells are more resistant to XR. At 6 Gy, the difference is of three-orders of magnitude. In the conditions tested, the difference of sensitivity between the two cell lines is more pronounced after XR, than after CPT addition.
Since the phosphorylation of NBS by ATM after irradiation has been largely documented (Gatei et al., 2000; Wu et al., 2000b; Zhao et al., 2000), we searched for evidence of this phosphorylation after CPT treatment (Figure 1b). Nuclear extracts were prepared in the presence of a cocktail of phosphatase inhibitors and the phosphorylated NBS was detected by Western blotting. A shift of the NBS position reflecting a retarded migration induced by the phosphorylation is already visible after 30 min and lasts for up to 2 h. This shift coincides with the shift observed after XR.
The relocalization of NBS in distinct patterns after γ-irradiation is also well described (Mirzeova and Petrini, 2001). After IR, the M/R/N nuclear foci are formed within 20 min, the number of the foci is maximal after 2 h and returns to normal after 24 h. When we followed the nuclear distribution of NBS in CPT-treated HeLa cells by immunofluorescence, we observed a different pattern of stability. As expected, NBS containing foci were visible 30 min after CPT addition (Figure 1c), and they were still visible after 24 h. The persistence of these foci could have resulted from the presence of CTP in the culture media for the complete duration of the incubation, by opposition to irradiation, which is limited in time and inflicts DNA damage during a shorter time frame. To answer that question, CPT was removed after 1 h of contact, and the cells were allowed to grow in fresh medium for an additional 23 h. It can be seen that, under these conditions, the nuclear foci are still visible 24 h after CPT addition (Figure 1c).
Next, we compared the cell cycle progression of NBS-ILB1 and NBS1-ILB1-6 cells after treatment with 1 μ M of CPT; CPT was either left in permanent contact with the cells (Figure 2a) or removed after 1 h (Figure 2b). The cytotoxicity observed was much higher when CPT was always kept in the culture media preventing us from following the cell cycle progression after 24 h. The cell cycle repartition was based upon the DNA content of the cells, which was measured by FACS after propidium iodide staining. When CPT at 1 μ M was left in permanent contact with the cells for 7 or 24 h, the absence of functional NBS only slightly affects the cellular repartition measured by FACS (Figure 2a). Indeed, the distribution of the cells between the G1, S and G2 phases was rather similar in the two cell lines at 7 h, but slight differences were observed after 24 h such as a higher S content. On the other hand, the cellular distribution was dramatically different between NBS-deficient and corrected cells when CPT was removed after 1 h of contact (Figure 2b). The corrected cells recovered a classical cell cycle distribution after 24 h, whereas the mutated cells still exhibited an aberrant profile after 24 and 48 h. Therefore, we can conclude that (i) the cells behave differently if CPT is left in permanent contact or washed away, a phenomenon that we could not detect when the nuclear foci stability was measured and (ii) NBS is actively involved in the cellular response initiated by CPT. However, this type of analysis does not allow us to conclude whether these cell cycle modifications are reflecting a default in DSB repair, in cell cycle control or in both.
Cell cycle modifications induced by CPT are NBS dependent. (a) NBS-ILB1 and NBS-ILB1-6 cells were treated with 1 μ M CPT for 7, 24 h. Cell cycle repartition of the cells based on their DNA content was measured by FACS with propidium iodide staining. (b) The same as in (a) except that CPT was removed after 1 h and the cells were subsequently grown for 7, 24 or 48 h in fresh media
In conclusion, the data presented above show that NBS participates in the cellular responses induced by DNA lesions that are generated by both XR and CPT. The cellular resistance to XR and CPT is decreased in NBS-deficient cells; NBS is rapidly phosphorylated after both types of treatment and its subnuclear localization is modified. The cell cycle distribution between NBS-deficient and proficient cells is different when cells are transiently exposed to CPT.
NBS participates in NF-κB activation after XR, but not after CPT
As a mutation in NBS decreases cell survival after DNA damage and since NF-κB is known to control genes encoding antiapoptotic proteins, we have monitored its induction in both NBS-ILB1 and NBS-ILB1-6 cells. The level of NF-κB induction after CPT or XR treatments was followed as a function of time by mobility shift assay. In parallel, NF-κB inducibility in each cell line was monitored by TNFα addition (Figure 3a, top panels). As both CPT and TNFα activate NF-κB via the IKK complex, an unaltered TNFα-elicited response establishes that the pathway downstream of the IKK complex is functional. The comparison between NBS-deficient and corrected cells showed that NF-κB induction following CPT addition is identical in both cell lines (Figure 3a, top panels). The kinetics of induction, as well as the level of activation are similar. The nature of the NF-κB subunits involved is also undistinguishable in both cell types; the complex being mostly made of p50/p65, with p52 and c-Rel after 4 h (Figure 3b, top panels). Since the cell cycle progression was clearly different if CPT was permanently left in contact with the cells or removed after 1 h, we repeated the previous experiments by removing CPT after 1 h of incubation. In this case, the kinetics of NF-κB activation were similar (data not shown) indicating that NF-κB induction by CPT is independent of NBS and rapidly initiated after CPT addition since an early removal of CPT does not affect the kinetics of activation.
NBS is involved in NF-κB activation after irradiation but not CPT treatment. (a) Comparison of NF-κB activation in NBS-ILB1 and NBS-ILB1-6. Both cell lines were either left untreated or treated with TNFα (200 U) for 30 min, CPT (10 μ M) as indicated (top panels), or irradiated (20 Gy) and then incubated for the indicated lengths of time (middle and bottom panels). Nuclear extracts derived from these cells were tested by EMSA for their ability to retard a 32P-radiolabelled κB probe, ‘ni’ indicates nonirradiated. (b) Nature of the complexes induced by CPT and XR in NBS-ILB1 and NBS-ILB1-6. Nuclear extracts from cells treated 4 h with CPT or irradiated and then incubated 7 h were incubated on ice for 15 min with antibodies directed against p50, p52, p65, c-rel or RelB. After that time, the radiolabelled probe was added and the incubation continued for an additional 30 min at room temperature
Following XR, a delay in NF-κB induction in NBS-ILB1 cells was observed compared to the control. NF-κB was already detectable in the nucleus after 30 min in the corrected cells, whereas it takes at least 90 min to be observable in NBS-ILB1 (Figure 3a, middle panels). A longer kinetic study reveals that NF-κB is induced by XR in NBS-deficient cells, but with a much lower intensity than in the corrected cells (Figure 3a, bottom panels). The nature of the NF-κB subunits identified by supershift with antibodies directed against p50, p52, p65, RelB and c-Rel is not affected by the NBS status. p50/p65 is the principal heterodimer found in both cell lines after 7 h (Figure 3b, bottom panels).
IκBα is partially degraded in both cell lines after either CPT or XR treatments and we could not detect any significant variation in the level of IκBβ and IκBɛ (data not shown).
hMRE11 is necessary for NF-κB activation after XR
In NBS-ILB1 cells, the absence of full-length NBS prevents the assembly of M/R/N in response to DNA damage, the nuclear foci are not formed and the hMRE11/RAD50 dimer is present in both the cytoplasm and nucleus (Carney et al., 1998). The effects described in the previous section could thus reflect the lack of functional M/R/N complex more than a specific role of the NBS protein by itself. To confirm the participation of the M/R/N complex in the signal transduction elicited by XR, we repeated the previous experiments with cells carrying a mutation in the hMRE11 gene. ATLD2 and ATLD3 are two different primary fibroblast cell lines containing different mutations of the hMRE11 gene (C1897 T and A350G leading to a truncation). Both mutations affect the efficiency of the heterotrimer assembly following DNA damage (Stewart et al., 1999). Once again, NF-κB activation by cytokines such as TNFα and IL1β is not affected in these cells. A strong signal is detected in the control cell line (CWAT) as well as in the two mutated cell lines: ATLD2 and ATLD3 (Figure 4, left panels). The addition of CPT (Figure 4, middle section) activates NF-κB at a similar level in the three different cell types regardless of the presence of hMRE11 mutations. The kinetics of induction are still slow and present a maximum at about 4 h confirming that M/R/N does not play a major role after CPT treatment as it was observed in the previous section. On the other hand, after XR, NF-κB activation is detected only in wild-type cells but not in ATLD2 and ATLD3 cells (Figure 4, right panels) demonstrating that the M/R/N trimer needs to be present in order to transduce the signal correctly. These data are in accordance with the results obtained using the NBS-ILB1 cells.
Mutation in hMRE11 significantly affects NF-κB activation after irradiation. The presence of NF-κB in nuclear extracts derived from CWAT, ATLD2 and ATLD3 primary fibroblasts treated by TNFα (200 U) or IL1β (100 U) for 30 min (left panels), by CPT (10 μ M) for the indicated length of time (middle panels), or XR (20 Gy) and then subsequently incubated for the indicated length of time (right panels) was determined by EMSA
BRCA1 modulates the NF-κB activation elicited by CPT and IR
Next, we tested the role of BRCA1 in the signal transduction initiated by CPT and XR. For this purpose, we used the HCC1937 cells that express a truncated form of BRCA1, whose last BRCT domain is lacking. Three different stimuli were used: TNFα (200 U/ml), CPT (10 μ M) and XR (20 Gy). As can be seen in the top two panels in Figure 5a, the kinetic of NF-κB activation by TNFα is similar in HCC1937 and HeLa cells, indicating that the steps of the cascade downstream of the IKK complex are equivalently functional. HCC1937 cells are more sensitive to IR than their corrected counterpart and do not form the typical BRCA1 foci after exposition to IR (Wu et al., 2000a) or CPT (data not shown). NF-κB activation after XR takes place quickly and is not sustained for long (Figure 5a, middle panels). This kinetic strongly differs from the one observed in HeLa cells. After CPT treatment (Figure 5a, bottom panels), we observed an opposite phenomenon: NF-κB induction is abnormally delayed in HCC1937 cells; an increase in NF-κB is still observed at 6 and 8 h. Altogether, these two observations indicate that wild-type BRCA1 is not essential for NF-κB activation by CPT or XR as it takes place even in its absence. However, the changes observed in the kinetics indicate that BRCA1 strongly modulates these responses. It should be noted that although a direct comparison between HCC1937 and HeLa cells is not ideal, it gives important information because HeLa cells expresses a full-length BRCA1 and is a model often used to study NF-κB activation.
Influence of BRCA1 in NF-κB activation by TNFα, CPT and XR. (a) Kinetics of NF-κB induction in HeLa and HCC1937 cells by TNFα, CPT and XR. HCC1937 cells (left panels) and HeLa cells (right panels) were treated with TNFα (200 U) (top panels) or with CPT (10 μ M) (bottom panels) for the indicated times. In the middle panels, the cells were irradiated with 20 Gy (1 Gy/min), then subsequently incubated at 37°C for the indicated times. After the incubation periods, the nuclear proteins were extracted and 5 μg was tested for their ability to retard 0.2 ng of 32P-radiolabelled oligonucleotide containing the κB consensus sequence. (b) Kinetics of degradation of IκBα in HCC1937 cells by the same stimuli. The levels of IκBα were monitored in 10 μg of cytoplasmic extracts of treated HCC1937 cells by Western blotting with an anti-IκBα antibody
Western blot analysis revealed that IκBα is extensively degraded after TNFα and XR treatments (Figure 5b). IκBα degradation after CPT was partial but still present. In a previous publication, we reported that in order to observe a strong IκBα degradation after CPT, HeLa cells must be synchronized in the S-phase when the drug is added (Habraken et al., 2001). The nature of the NF-κB subunit was identified at maximum induction (30 min for XR and 8 h for CPT) and only p50 and p65 were detected (data not shown).
MMR pathway is essential to NF-κB activation after both CPT and XR
It has been previously reported that MMR-deficient cells are more sensitive to CPT (Jacobs et al., 2001; Pichierri et al., 2001). We then decided to compare HCT116 cells, which are human colon carcinoma cells lacking both MLH1 and MSH6, with their corrected isogenic counterpart HCT116 3-6. In the latter, the chromosome 3 carrying the MLH1 gene has been reintroduced (Koi et al., 1994), and although the cells are not corrected for MSH6, the MMR system is restored as many of the MSH6 functions can be carried out by MSH3. The re-expression of MLH1, in nuclear extracts derived from HCT116 3-6, was confirmed by Western blotting (Figure 6a, inset). The resistance of HCT116 and HCT116 3-6 cells to CPT was measured by clonogenic assay. As expected, a small but significant increase in the survival fraction of the corrected cells was observed (Figure 6a). In order to ascertain that both cell lines experienced a similar level of DNA damage, we monitored the p53 stabilization after CPT treatment. Western blot analysis reveals that the level of p53 increased similarly in deficient and corrected cells, although the basal level of p53 is slightly higher in the deficient cell line (Figure 6b). The comparable p53 stabilization in both cell lines, reflects a similar level of DNA damage. Next, we compared NF-κB induction in MMR-deficient and proficient cells by XR, CPT and cytokines (Figure 6c). We observed that NF-κB is very efficiently induced by TNFα and IL1β in HCT116 cells confirming that the pathway downstream of the IKK complex is operating correctly within that genetic background. On the other hand, when DBS were introduced either by CPT or XR, no NF-κB activation was detected in HCT116 cells. The NF-κB activation by both stimuli was restored in the corrected HCT116 3-6 cells indicating that MLH1 is playing a role in the transduction cascade initiated by DSB. Taken together, these data indicate that a proficient MMR pathway is essential for NF-κB activation following XR and CPT treatments.
A functional MMR system is essential CPT or XR-related NF-κB activation. (a) Survival of HCT116 and HCT116 3-6 cells after CPT treatment. Both cell lines were plated at clonal concentration and then treated with 0, 5, 10, 20 or 30 nM CPT in complete media for 24 h; after replacement with fresh media, the cells were grown for an additional 14 days before fixation and coloration. The colonies present on each plate were counted and expressed as a percentage of the nontreated cells. Each point represents the mean value of two independent experiments with triplicates (±s.d.). HCT116 (open diamond), HCT116 3-6 (filled squares). Inset: Western blot analysis of 10 μg nuclear extracts derived from HCT116 and HCT116 3-6 probed with hMLH1 antibodies, ns means nonspecific. (b) CPT induces the stabilization of p53 independently of MMR. The level of p53, in 10 μg nuclear extracts derived from HCT116 and HCT116 3-6 cells treated with CPT (10 μ M) for the increasing length of time was monitored by Western blot analysis. (c) Functional MMR is essential for NF-κB activation by XR and CPT, but not by TNFα. EMSA with nuclear extracts derived from HCT116 or HCT116 3-6 cells treated with TNFα (200 U), IL1β (100 U) for 30 min or treated with CPT (10 μM) for the indicated period of time (top panels) or irradiated (20 Gy) and subsequently incubated for increasing lengths of time (bottom panels). (d) Full-length hMRE11 is expressed in HCT116. hMRE11 level was compared in ATLD2, HCT116, HCT116 3-6 and HeLa cells (left panel) and the level of NBS was compared in NBS-ILB1, HCT116, HCT116 3-6 and HeLa cells (right panel), ns means nonspecific
Recently, Giannini et al. reported that HCT116 cells do not express hMRE11. However, we found that the level of hMRE11 in the nucleus of HCT116 and HCT116 3-6 cells is reduced in comparison with the level found in HeLa cells but not null (Figure 6d). ATLD2 cells that do not express any full-length Mre11 serve as a negative control. To a lesser extent, the same observation holds true for NBS. NBS levels are lower in HCT116 and HCT116 3-6 than in HeLa cells. For comparison purposes, we added an extract from NBS-ILB1 cells, which do not contain any full-length NBS. Thus, Western blot analysis indicates that the M/R/N level is attenuated in HCT116 and HCT116 3-6 cells but not null. As we have shown above that the M/R/N trimer is indispensable to the NF-κB activation after XR, this low level of M/R/N must be sufficient to transduce the signal.
XPF does not participate in NF-κB activation elicited by CPT
In Saccharomyces cerevisiae, the trapped CPT-Topo-1 cleavage complex can be removed from the collapsed replication forks by three different pathways involving Tdp1 (tyrosyl-DNA phosphodiesterase) or the Rad1-Rad10 complex or Mus81, respectively (Vance and Wilson, 2002; Liu et al., 2002). Independent from its function in the nucleotide excision repair system, the Rad1-Rad10 complex is able to remove the nonhomologous tail during recombination processes. In our search to identify the element initiating the signal transduction elicited by CPT, we analysed NF-κB activation in XPF cells. XPF is the human homolog of Rad1. As can be seen in Figure 7, NF-κB is activated at a similar level in immortalized XPF compared to normal fibroblasts demonstrating that XPF has no role in the NF-κB transduction pathway induced by CPT.
Discussion
The cellular processing of DSB is complex and involves both cell cycle regulation and repair by HR and NHEJ. In this work, two types of DSB were introduced: CPT-related breaks that result from the collision of replication forks with the trapped Topo-I complex during the S-phase and XR-related breaks that are created independent of the cell cycle phase. The ‘extremities’ of the breaks are different: exclusively 5′-OH and 3′-phosphotyrosine enzyme–DNA intermediates for CPT versus sugar fragments for XR. Moreover, the broken strands generated by CPT are completely homologous with their sister chromatids and thus, during the recombination process, there should not be any nonhomologous tail to remove. As it was previously established that the signal transduction between DNA breaks and NF-κB is ATM dependent (Piret et al., 1999, Li et al., 2001), we studied three substrates of ATM that play a role in HR and/or NHEJ as well as in cell cycle control. All the cell lines used in this study were able to activate NF-κB after TNFα or IL1β treatment, indicating that steps in the pathway downstream of the IKK complex are functional. Our data demonstrated that the M/R/N complex has no role in this transduction when DSB are generated by CPT. This was unexpected since NBS is involved in many aspects of the CPT-induced response. Following the addition of CPT, (i) NBS is rapidly phosphorylated, (ii) NBS containing nuclear foci are formed and are stable, (iii) NBS increases the cellular resistance and affects the cell cycle distribution. Nevertheless, neither the kinetic nor the level of activation or the nature of the subunits forming the NF-κB complex were modified in the absence of NBS. These data were confirmed with ATLD2 and ATLD3 cells that still show a classical activation of NF-κB after CPT treatment, although they do not express hMRE11 and consequently lack the M/R/N trimer.
Different results were obtained when the DSB were generated by XR. NF-κB activation was delayed and attenuated in NBS-deficient cells and abrogated in hMRE11-deficient cells. Although the signal is weak, it was possible to determine the nature of the NF-κB subunits in NBS-ILB1 cells; they are identical in both NBS-ILB1 and NBS-ILB1-6 cell lines, but different from the one observed after CPT treatment. The weak residual activation in NBS cells could result from the partial substitution of p96 by p70 (a smaller form of NBS presents in minute amount in NBS-ILB1 cells) and therefore minimizing the observed effects.
From these observations and others previously published, we can conclude that (i) at least two different pathways can transduce the signal originating from DSB: the CPT-related pathway that is M/R/N independent and the XR-related pathway that is M/R/N dependent, (ii) these two pathways are ATM dependent as both are affected in AT cells, (iii) NF-κB activation is initiated rapidly after CPT addition because the removal of the drug after 1 h of contact does not modify the kinetic of activation, whereas it profoundly modifies the cell cycle repartition of the cells, and (iv) nuclear foci formation and stability are not related to CPT-induced activation of NF-κB. Very recently, it was shown that HA2X is rapidly phosphorylated by ATR and DNA-PK after CPT addition and that γHA2X is indispensable to the formation of N/M/R foci (Furuta et al., 2003). ATM may have a contributory role since the level of γH2AX is reduced in AT cells. Therefore, one can make the hypothesis that CPT could rapidly activate ATR, which in turn initiates the cascade leading to γH2AX and NBS foci formation. However, these recent observations do not preclude that ATM initiates the cascade leading to IKK activation by CPT and XR.
As with M/R/N, the role of BRCA1 is not yet fully understood. BRCA1 participates in many important aspects of the response to DSB, in the genome surveillance and in the cell cycle arrest. Our data indicate that the kinetics of NF-κB activation are atypical in BRCA1-deficient cells, NF-κB activation occurred early after XR and is delayed after CPT treatment. BRCA1 is known to bind branched structures (Paull et al., 2000) and by doing so interferes with the endonuclease activity of M/R/N. Thus, BRCA1 could delay the function of M/R/N after XR and then delays NF-κB activation. Although it is still unknown how exactly BRCA1 proceeds, its mutation alters NF-κB induction differently after CPT and XR, therefore reinforcing the idea that two different pathways are involved in NF-κB activation by DSB.
Since the establishment of the corrected HCT116 cells by introduction of chromosome 3 in the deficient cells, the tandem HCT116/HCT116 3-6 has often been used to compare MMR-deficient and -proficient cells. Our results indicate that an efficient MMR pathway is required for NF-κB activation after DSB. One study has reported that HCT116 cells do not express hMRE11 (Giannini et al., 2002), whereas a second one could not confirm the reduced level of expression of this protein (Brown et al., 2003). Because in this work we have shown that the XR-related signal was M/R/N dependent, it was important to verify the level of hMRE11 in these cells. We found a reduced but still significant level of hMRE11. NBS level was also lower confirming that the three members of the trimer are required for its maximal stability. Apparently, this small amount of M/R/N is still able to transduce the signal. Indeed, if it were not so, we would not have observed a restoration of the NF-κB activation after XR in HCT116 3-6 cells. hMLH1, like ATM, affects both M/R/N-independent and -dependent pathways. ATM is activated very early in the cascade initiated by DSB. MMR, which controls S-phase checkpoint activation and acts during HR, could transduce the signal at two different moments, either very early at the level of the breaks in association with ATM when establishing the cell cycle control or later at the level of the Holliday junction during the repair step. The association of ATM and hMLH1 was detected both in vivo and in vitro (Brown et al., 2003), indicating that the mismatch repair complex can be formed at the site of DNA damage and favouring the first hypothesis.
In summary, NF-κB activation by DSB can be mediated by two nuclear pathways. The CPT-induced cascade would transit via ATM, MMR and is positively modulated by BRCA1. The XR-induced cascade would occur via ATM, MMR and M/R/N, and is negatively regulated by BRCA1. The exact role of hMLH1 and M/R/N in these two transduction signalling pathways is under further investigations. Actually, we do not know whether the NF-κB activation results from their participation in the cell cycle control or their implication in the DSB repair by HR or NHEJ.
NF-κB is an antiapoptotic factor and a higher sensitivity of cells to DNA damage is then expected when its induction is lowered. Such a correlation is seen in XR-treated NBS-and hMRE11-deficient cells, but not in the CPT-treated cells or with the MMR-deficient cells indicating that other factors might regulate the sensitivity to genotoxic agents.
Materials and methods
Cell lines and chemicals
HeLa cells were cultivated in EMEM with 10% foetal calf serum (Biowhittaker, Petit-Rechain, Belgium). Immortalized fibroblasts NBS-ILB1 and NBS-ILB1 infected with an NBS retrovirus (NBS-ILB1-6) were kindly supplied by Dr Zdzienicka (Leiden University, Netherlands) and Dr Concannon (Virginia Mason Research Center, Seattle, USA), respectively. They were grown in DMEM complemented with F12 salts and 10% foetal calf serum (Invitrogen, USA). Colon cancer HCT116 and corrected HCT116 3-6 (received from Dr Boland, University of California, La Jolla, USA) were grown in DMEM and 10% foetal calf serum. G418 (Invitrogen) at 500 μg/ml was added to the media of both corrected cells. Skin fibroblasts CWAT, ATLD2, ATLD3 (received from Dr Taylor, CRC Institute for Cancer Research, Birmingham, UK), the ductal breast carcinoma HCC1937 cells (ATCC), XPF−/− fibroblasts (GM08437A, ATCC) and normal fibroblasts (GM006376, ATCC) were all cultivated in DMEM with high glucose and 10% foetal calf serum. All the cells were grown at 37°C under a 5% CO2 atmosphere without penicillin or streptomycin. CPT was purchased from Sigma (St Louis, USA) and dissolved in DMSO. Recombinant human TNFα and IL1β were obtained from Roche (Mannheim, Germany).
XR treatment
Cells were irradiated at room temperature in complete media using a Stabilivolt (Siemens, Germany) operated at 190 kV, 18 mA with a 0.5 mm Cu filter at such a distance that the dose rate was 1 Gy/min. A calibrated in-field ionizing monitor was used at each irradiation to ensure the accuracy of the delivered dose. HCT116, HCT116 3-6, CWAT, ATLD2 and ATLD3 cells were placed in DMEM media complemented with F12 salt the day prior to the irradiation.
Cell viability determined by colony-forming assay
Cell sensitivity to death by CPT or XR was determined by clonogenic assay. For survival after CPT treatment, deficient and corrected cells were plated at different densities (from 100 to 80 000 cells/dish) the day prior to the addition of the drug. CPT at 0, 5, 10, 20 or 30 nM was added and left in contact with the cells for 24 h. After that time, all dishes were washed twice and the cells were left to grow for 10–14 days in complete media. The clones were fixed and coloured before counting. For survival after irradiation, deficient and corrected cells were plated at subconfluence the day prior to the irradiation. After exposition to 0, 2, 4 or 6 Gy, the cells were trypsinized, plated at different densities and left to grow for 10–14 days in complete media. The colonies were then fixed and coloured before counting.
Cytoplasmic and nuclear protein extracts preparation
Cells at 70–80% of confluence in complete media were treated with CPT (10 μ M) or exposed to XR (20 Gy at 1 Gy/min). After the indicated times post treatment, the cells were washed twice with ice-cold PBS, scraped and centrifuged. The cytoplasmic and nuclear extracts were prepared as previously described (Habraken et al., 2001). The protein concentration was determined with Bio-Rad protein assay. When needed, phosphatase inhibitors Na3VO4 (1 mM), NAF (1 mM), β-glycerophosphate (1 mM) and p-nitro-phenylphosphate (1.5 mM) were added to both the cytoplasmic and nuclear extraction buffers.
Western blots, electrophoretic mobility assay and supershift
The levels or the presence of IκBα, IκBβ, IκBɛ, NBS, phosphorylated-NBS, MLH1 and p53 were determined by Western blottings performed with 10 μg of nuclear or cytoplasmic extracts with appropriate antibodies. Monoclonal anti-IκBα antibodies were donated by C Dargemont (Institute J Monot, Paris). Antibodies anti-NBS and -MLH1 were purchased from Becton and Dickinson BENELUX. Antibodies anti-p53, -hMRE11, -BRCA1 originated from Oncogene. Antibodies anti-ATM, -IκBβ, -IκBɛ were from Santa Cruz Biotechnology. Secondary antibodies coupled to HRP were from DakoCytomation (Glostrup, Denmark).
The binding reactions and supershift experiments were performed with 10 μg of nuclear proteins and 0.2 ng κB probe as previously described (Schoonbroodt et al., 2000; Habraken et al., 2001). The antibodies used in the supershift experiment directed against p50, p52, p65, c-rel or relB were all bought from Santa Cruz Biotechnology.
Immunofluorescence analysis
Cells grown on glass coverslips were either treated with CPT (10 μ M) or not and incubated 15 min to 24 h at 37°C to allow foci formation. In some instances, CTP was removed after 1 h and the drug-containing media were replaced by fresh media. The cells were fixed 20 min at −20°C with acetone/methanol (v/v) and air-dried. The coverslips were then incubated in PBS containing powdered skimmed milk (1.5%) at room temperature for 30 min. After washing, PBS containing anti-NBS antibody was poured over the fixed cells and incubated at 37°C for 45 min. The coverslips were washed three times for 10 min with PBS and incubated in PBS containing Evans blue at 1% (v/v) to counterstain the cells and secondary antibody coupled to fluorescein isothiocyanate for 45 min at 37°C. After the incubation, the cells were washed in three very large volumes of water before being mounted with Fluoroprep on microscope slides. Cellular immunofluorescence was analysed using a Nikon Fluorescence Microscope. Foci were visualized at a magnification of × 1000 under oil immersion.
Cell cycle analysis
Cell cycle distributions were analysed with flow cytometry. At the indicated times after CPT treatment, the cells were prepared for analyses with the Cycle TestTM plus DNA reagent kit (Becton and Dickinson). The DNA content of the cells was determined with a FACS calibur (Becton and Dickinson) and the data were treated with WinMDI 2.8 program.
References
Avemann K, Knippers R, Koller T and Sogo J . (1998). Mol. Cell. Biol., 8, 3026–3024.
Bharti A and Aggarwal B . (2002). Biochem. Pharmacol., 64, 883–888.
Brown K, Rathi A, Kamath R, Beardsley D, Zhan Q, Mannino J and Baskaran R . (2003). Nat. Genet., 33, 80–84.
Carney J, Maser R, Olivares H, Davis E, Le Beau M, Yates III J, Hayes L, Morgan W and Petrini J . (1998). Cell, 93, 477–486.
Cerosaletti K, Desai-Mehta A, Yeo T, Kraakman-Van Der Zwet M, Zdzienicka M and Concannon P . (2000). Mutagenesis, 15, 281–286.
Cortez D, Wang Y, Qin J and Elledge S . (1999). Science, 286, 1162–1166.
D'Amours D and Jackson S . (2002). Nat. Rev., 3, 317–327.
Friedberg EC, Walker JGC and Siede W . (1995). DNA Repair and Mutagenesis. ASM Press: Washington, DC.
Furuta T, Takemura H, Liao Z-Y, Aune G, Redon C, Sedelnikova O, Pilch D, Rogakou E, Celeste A, Chen H, Nussenzweig A, Aladlem M, Bonner W and Pommier Y . (2003). J. Biol. Chem. 278, 20303–20315.
Gatei M, Young D, Cerosaletti K, Desai-Mehta A, Spring K, Kozlov S, Lavin M, Gatti R, Concannon P and Khanna K . (2000). Nat. Genet., 25, 115–119.
Gosh S and Karin M . (2002). Cell, 109 (Suppl.), S81–S96.
Giannini G, Ristori E, Cerignoli F, Rinaldi C, Zani M, Viel A, Ottini L, Crescenzi M, Martinotti S, Bignami M, Frati L, Screpanti I and Gulino A . (2002). EMBO Rep., 3, 248–254.
Habraken Y, Piret B and Piette J . (2001). Biochem. Pharmacol., 62, 603–616.
Harfe B and Jinks-Robertson S . (2000). Annu. Rev. Genet., 34, 359–399.
Huang T, Wuerzberger-Davis S, Seufzer B, Shumway S, Kurama T, Boothman D and Miyamoto S . (2000). J. Biol. Chem., 275, 9501–9509.
Hur G, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S and Liu Z . (2003). Gene Dev., 17, 873–882.
Jacob S and Praz F . (2002). Biochimie, 84, 27–47.
Jacob S, Aguado M, Fallik D and Praz F . (2001). Cancer Res., 61, 6555–6562.
Karin M, Cao Y, Greten F and Li Z . (2002). Nat. Rev. Cancer, 2, 301–310.
Koi M, Umar A, Chauhan D, Cherian S, Carethers J, Kunkel T and Boland C . (1994). Cancer Res., 54, 4308–4312.
Kraakman-Van Der Zwet M, Overkamp W, Friedl A, Klein B, Verhaegh G, Jaspers N, Midro A, Eckardt-Schupp F, Lohman P and Zdzienicka M . (1999). Mutat. Res., 434, 127.
Li N, Banin S, Ouyang H, Li G, Courtois G, Shiloh Y, Karin M and Rotman G . (2001). J. Biol. Chem., 276, 8898–8903.
Lim D-S, Kim T, Xu B, Maser R, Lin J, Petrini J and Kastan M . (2000). Nature, 404, 613–617.
Liu C, Pouliot J and Nash H . (2002). Proc. Natl. Acad. Sci., 99, 14970–14975.
Mirzeova O and Petrini J . (2001). Mol. Cell. Biol., 21, 281–288.
Paull T, Cortez D, Bowers B, Elledge S and Gellert M . (2001). Proc. Natl. Acad. Sci., 98, 6086–6091.
Petrini J . (2000). Curr. Opin. Cell Biol., 12, 293–296.
Pichierri P, Franchitto A, Piergentili R, Colussi C and Palitti F . (2001). Carcinogenesis, 22, 1781–1787.
Piret B and Piette J . (1996). Nucleic Acid Res., 24, 4242–4248.
Piret B, Schoonbroodt S and Piette J . (1999). Oncogene, 18, 2261–2271.
Pommier Y, Pourquier P, Fan Y and Strumberg D . (1998). Biochim. Biophys. Acta, 1400, 83–105.
Shiloh Y . (1997). Ann. Rev. Genet., 31, 635–662.
Scully R, Ganesan S, Vlasakova K, Chen J, Socolovsky M and Livingston D . (1999). Mol. Cell, 4, 1093–1097.
Stewart G, Maser R, Stankovic T, Bressan D, Kaplan M, Jaspers N, Raams A, Byrd P, Petrini J and Taylor A . (1999). Cell, 99, 577–587.
Thompson L and Schild D . (2002). Mut. Res., 509, 49–78.
Vance J and Wison T . (2002). Proc. Natl. Acad. Sci., 99, 13669–13674.
Varon R, Vissinga C, Platzer M, Cerosaletti K, Chrzanowska K, Saar K, Beckmann G, Seemanova E, Cooper P, Nowak N, Stumm M, Weemaes C, Gatti R, Wilson R, Digweed M, Rosenthal A, Sperling K, Concannon P and Reis A . (1998). Cell, 93, 467–476.
Venkitaraman A . (2001). J. Cell Sci., 114, 3591–3598.
Xu B, Kim S, Lim D and Kastan M . (2002). Mol. Cell. Biol., 22, 1049–1059.
Wu X, Petrini J, Heinse W, Weaver D, Livingston D and Chen J . (2000a). Science, 289, 11a.
Wu X, Ranganathan V, Weisman D, Heine W, Ciccone D, O'Neill T, Crick K, Pierce K, Lane W, Rathbun G, Livingston D and Weaver D . (2000b). Nature, 405, 477–482.
Zhao S, Weng Y, Yuan S, Lin Y, Hsu H, Lin S, Gerbino E, Song M, Zdzienicka M, Gatti R, Shay J, Ziv Y, Shiloh Y and Lee E . (2000). Nature, 405, 473–477.
Zhong Q, Boyer T, Chen P and Lee W . (2002). Cancer Res., 62, 3966–3970.
Zhong Q, Chen C, Li S, Chen Y, Wang C-C, Xiao J, Chen P-L, Sharp Z and Lee W . (1999). Science, 285, 747–750.
Acknowledgements
We are indebted to Dr M Zdzienicka (Leiden University, Netherlands) who kindly supplied us NBS-ILB1 cells and to Dr P Concannon (Virginia Mason Research Center, Seattle, USA) who sent us NBS-ILB1-6 cells. CWAT, ATLD2 and ATLD3 cells were a generous gift from Dr M Taylor (University of Birmingham, CRC Institute for Cancer Research, Birmingham, UK). We also thank Dr R Boland (UCSF, La Jolla, USA) for allowing us to use his corrected HCT116 3-6 cells. We thank Ch De Jesus for his assistance in this work. This work was supported by grants from the Belgian National Fund for Scientific Research (NFSR, Brussels, Belgium), from an IAP program (P5/12) and from a starting grant from the University of Liège. YH is a Research Scientist and JP is a Research Director, both supported by the NFSR. OJ is an assistant from the University of Liège.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habraken, Y., Jolois, O. & Piette, J. Differential involvement of the hMRE11/hRAD50/NBS1 complex, BRCA1 and MLH1 in NF-κB activation by camptothecin and X-ray. Oncogene 22, 6090–6099 (2003). https://doi.org/10.1038/sj.onc.1206893
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206893
Keywords
This article is cited by
-
Alteration in stemness causes exclusivity between Epstein–Barr virus-positivity and microsatellite instability status in gastric cancer
Gastric Cancer (2021)
-
DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer
Signal Transduction and Targeted Therapy (2020)
-
NF-κB is a critical mediator of BRCA1-induced chemoresistance
Oncogene (2014)