Abstract
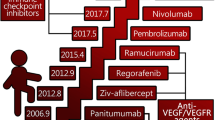
In less than 10 years, the number and importance of non-surgical treatment modalities in patients with colorectal cancer (CRC) have increased dramatically, both in the adjuvant and the advanced settings. However, despite the improvement of cytotoxic therapy in CRC, many patients still develop progressive disease and unfortunately in patients with disease resistant to 5-fluorouracil/folinic acid, irinotecan and oxaliplatin, no effective cytotoxic therapy is known. The rapidly expanding knowledge in tumor biology has encouraged optimism for the possibility to find and target tumor-specific mechanisms and thereby increase both efficacy and tolerance. A great number of ‘targeted drugs’ are being tested in clinical trials and some of these new drugs, like bevacizumab, cetuximab and panitumumab, are available for routine use in health care. These new targeted drugs will expand the therapeutic arsenal in CRC to a great extent, but they will also add to the complexity of treatment of CRC. In this review, we summarize the current status of antibody therapy in patients with CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Abubakr Y, Eng C, Wong L, Pautret V, Scheithauer W, Maurel J et al. (2006). Cetuximab plus irinotecan for metastatic colorectal cancer (mCRC): Safety analysis of 800 patients in a randomized phase III trial (EPIC). J Clin Oncol 24 (18S): Abstract 3556.
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D et al. (2004). Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy. A model to predict long-term survival. Ann Surg 240: 644–658.
Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK et al. (2001). High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 61: 4750–4755.
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC . (2006). Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 55: 657–670.
Andre T, Tabernero J, Van Cutsem E, Diaz-Rubio E, Cervantes A, Humblet Y et al. (2007). Phase II study with cetuximab plus FOLFOX-4 in first-line setting for epidermal growth factor receptor (EGFR)-expressing metastatic colorectal cancer (mCRC): Final results. Proc ASCO GI: abstract 334.
Arnold D, Siewczynski R, Schmoll HJ . (2006). The integration of targeted agents into systemic therapy of metastatic colorectal cancer. Ann Oncol 17: 122–128.
Azuma M, Danenberg KD, Iqbal S, El-Khoueiry A, Zhang W, Yang D et al. (2006). Epidermal growth factor receptor and epidermal growth factor receptor variant III gene expression in metastatic colorectal cancer. Colorectal Cancer 6: 214–218.
Barber TD, Vogelstein B, Kinzler KW, Velculescu VE . (2004). Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med 351: 2883.
Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M et al. (2000). Phase I studies of anti epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol 18: 904–914.
Berry SR, Cunningham D, Michael M, Dibartolomeo M, Rivera F, Kretzschmar A et al. (2006). Preliminary safety of bevacizumab with first-line Folfox, Capox, Folfiri and capecitabine for mCRC-First BEA Trial. Proc ASCO GI: abstract 3534.
Borner M, Mingrone W, Koeberle D, Von Moos R, Rauch D, Saletti P et al. (2006). The impact of cetuximab on the capecitabine plus oxaliplatin (XELOX) combination in first-line treatment of metastatic colorectal cancer (MCC): a randomized phase II trial of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol 24: abstract 3551.
Bouché O, Brixi-Benmansour H, Bertin A, Perceau G, Lagarde S . (2005). Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol 16: 1711–1712.
Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC . (2001). Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144: 1169–1176.
Carmichael J, Popiela T, Radstone D, Falk S, Borner M, Oza A et al. (2002). Randomised comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20: 3617–3627.
Carson EJ, Novak AM, Stella PJ . (2005). Hypomagnesemia in patients with stage IV colorectal cancer treated with cetuximab as a single agent. J Clin Oncol 23: abstract 3655.
Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R et al. 2006)). First efficacy and safety results from XELOX-1/NO16968, a randomized 2 × 2 factorial phase III trial of XELOX vs FOLFOX4+bevacizumab or placebo in first-line metastatic colorectal cancer (MCRC). AnnOncol 17: abstract LBA3.
Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L et al. (2006). Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI treatment referral center trial TRC-0301. J Clin Oncol 24: 3354–3360.
Chong D, Bhatnagar A, Cunningham D, Cosgriff TM, Harper PG, Steward W et al. (2006). Phase III trial of 5-fluorouracil and leucovorin plus either 3H1 anti-idiotype monoclonal antibody or placebo in patients with advanced colorectal cancer. Ann Oncol 17: 437–442.
Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A et al. (2005). Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23: 1803–1810.
Ciardiello F, Tortora G . (2003). Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer 39: 1348–1354.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al. (2004). Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351: 337–345.
Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R et al. (1998). Randomised trial of iriontecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352: 1413–1418.
Cunningham MP, Thomas H, Fan Z, Modjtahedi H . (2006). Responses of human colorectal tumor cells to treatment with the anti–epidermal growth factor receptor monoclonal antibody ICR62 Used alone and in combination with the EGFR tyrosine kinase inhibitor gefitinib. Cancer Res 66: 7708–7715.
de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL et al. (1997). Randomised trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15: 808–815.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J et al. (2000). Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18: 2938–2947.
Dittrich C, Hoehler T, Lordick F, Seufferlein T, Riemann J, Woell E et al. (2006). A phase I/II study of cetuximab in combination with 5-fluorouracil (5-FU)/folinic acid (FA) plus weekly oxaliplatin (L-OHP) (FUFOX) in the first-line treatment of patients with metastatic colorectal cancer (mCRC) expressing epidermal growth factor receptor (EGFR). Preliminary results. Ann Oncol 17: abstract 019.
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P et al. (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355: 1041–1047.
Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P et al. (2002). Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20: 3605–3616.
Ellis LM, Curley SA, Grothey A . (2005). Surgical resection after downsizing of colorectal liver metastasis in the era of bevacizumab. J Clin Oncol 23: 4853–4855.
Fakih MG, Wilding G, Lombardo J . (2006). Cetuximab-induced hypomagnesimia in patients with colorectal cancer. Clin Colorectal Cancer 6: 152–156.
Falcone A, Masi G, Brunetti I, Benedetti G, Bertetto O, Picone V et al. (2006). The triplet combination of irinotecan, oxaliplatin and 5FU/LV (FOLFOXIRI) vs the doublet of irinotecan and 5FU/LV (FOLFIRI) as first-line treatment of metastatic colorectal cancer (MCRC): results of a randomized phase III trial by the Gruppo Oncologico Nord Ovest (GONO). J Clin Oncol 24: abstract 3513.
Ferrara N . (2004). Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 4: 581–611.
Folprecht G, Lutz MP, Schöffski P, Seufferlein T, Nolting A, Pollert P et al. (2006). Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol 17: 450–456.
Folprecht NG, Grothey A, Alberts S, Raab H-R, Kohne C-H . (2005). Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 16: 1311–1319.
Fuchs C, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M et al. (2006). A randomized trial of first-line irinotecan/fluoropyrimidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C). J Clin Oncol 24: abstract 3506.
Fyfe GA, Hurwitz H, Fehrenbacher L, Cartwright T, Hainsworth J, Heim W et al. (2004). Bevacizumab plus irinotecan/5-FU/leucovorin for treatment of metastatic colorectal cancer results in survival benefit in all prespecified patient subgroups. J Clin Oncol 22: abstract 3617.
Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C et al. (2000). Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18: 136–147.
Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR et al. (2005). High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the Eastern Cooperative Oncology Group (ECOG) study E3200. J Clin Oncol 23: Abstract 2.
Giantonio BJ, Catalano PJ, O'Dwyer PJ, Meropol NJ, Benson AB . (2006b). Impact of bevacizumab dose reduction on clinical outcomes for patients treated on the Eastern Cooperative Oncology Group's Study E3200. J Clin Oncol 24: abstract 3538.
Giantonio BJ, Levy DE, O'dwyer PJ, Meropol NJ, Catalano PJ, Benson III AB . (2006a). A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: results from the Eastern Cooperative Oncology Group study E2200. Ann Oncol 17: 1399–1403.
Gibson TB, Ranganathan A, Grothey A . (2006). Randomized phase III trial resaults of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer 6: 29–31.
Glimelius B, Sorbye H, Balteskard L, Byström P, Pfeiffer P, Tveit KM et al. (2005). A randomised phase III multicenter trial comparing irinotecan in combination with either the Nordic bolus 5FU and folinic acid (5FU/FA) schedule (FLIRI) or the bolus/infused de Gramont schedule (FOLFIRI), in patients with metastatic colorectal cancer. Eur J Cancer Suppl 3: abstract: 597.
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK et al. (2004). A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22: 23–30.
Grabau D, Nielsen O, Hansen S, Nielsen MM, Laenkholm AV, Knoop A et al. (1998). Influence of storage and high-temperature antigen retrieval buffers on results of immunohistochemical staining in sections stored for long periods. Appl Immunohistochem 6: 209–213.
Grothey A, Deschler B, Kroening H, Ridwelski K, Reichardt P, Kretzschmar A et al. (2002). Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24 h 5-FU infusion/FA+oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC). Proc Am Soc Clin Oncol 21: abstract 512.
Grothey A, Jordan K, Kellner O, Constantin C, Dittrich G, Kroening H et al. (2003). Capecitabine plus irinotecan (CAPIRI) vs capecitabine plus oxaliplatin (CAPOX) as first-line therapy of advanced colorectal cancer (ACRC): updated results of a randomized phase II trial. Eur J Cancer 90: abstract 295.
Grothey A, Sargent D . (2005). Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol 23: 9441–9442.
Grothey A, Sargent D, Goldberg RM, Schmoll HJ . (2004). Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil–leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22: 1209–1214.
Gruenberger T, Gruenberger B, Scheithauer W . (2006). Neoadjuvant therapy with bevacizumab. J Clin Oncol 24: 2592–2593.
Hambleton J, Novotny WF, Hurwitz H, Fehrenbacher L, Cartwright T, Hainsworth J et al. (2004). Bevazicumab does not increase bleeding in patients with metastatic colorectal cancer receiving concurrent anticoagulation. J Clin Oncol 22: abstract 3528.
Hambleton J, Skillings J, Kabbinavar F, Bergsland E, Holmgren E, Holden SN et al. (2005). Safety of low-dose aspirin (ASA) in a pooled analysis of 3 randomized, controlled trials (RCTs) of bevacizumab (BV) with chemotherapy (CT) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 23: abstract 3554.
Hanahan D, Weinberg RA . (2000). The hallmarks of cancer. Cell 100: 57–70.
Harari PM, Durland WF, Chinnaiyan P . (2003). Impact of the EGFR inhibitor C225 on wound healing in advanced head and neck cancer patients undergoing neck dissection. Proc Am Soc Clin Oncol 22: abstract 881.
Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP et al. (2006). Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anti-Cancer Drugs 17: 855–857.
Hecht J, Mitchell E, Baranda J, Malik I, Richards D, Navale L et al. (2006b). Panitumumab antitumor activity in patients with metastatic colorectal cancer expressing low (1–9%) or negative levels of EGFR. J Clin Oncol 24: abstract 3547.
Hecht J, Posey J, Tchekmedyian S, Hu E, Chan D, Malik I et al. (2006a). Panitumumab in combination with irinotecan, 5-flurouracil, and leukovorin (IFL) or FOLFIRI for first-line treatment of metastatic colorectal cancer. Proc ASCO GI: abstract 237.
Hedrick E, Hurwitz H, Sarkar S, Griffing S, Novotny W, Grothey A . (2004). Post-progression therapy (PPT) effect on survival in AVF2107, a phase III trial of bevacizumab in first-line treatment of metastatic colorectal cancer (mCRC). J Clin Oncol 22: abstract 3517.
Heinemann V, Fischer Von Weikersthal L, Moosmann N, Vehling-Kaiser U, Stauch M, Oruzio D et al. (2006). Cetuximab+capecitabine+irinotecan (CCI) versus cetuximab+capecitabine+oxaliplatin (CCO) as first line therapy for patients with metastatic colorectal cancer (CRC): preliminary results of a randomized phase II trial of the AIO CRC Study Group. J Clin Oncol 24: abstract 3550.
Hicklin DJ, Ellis LM . (2005). Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23: 1011–1027.
Hillner BE, Schrag D, Sargent DJ, Fuchs CS, Goldberg RM . (2005). Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer 104: 1871–1884.
Ho C, Eisen D, Beckett L, Luciw P, Gumerlock PH, Gandara DR et al. (2006). Escalating weekly doses of cetuximab: a Phase I trial in advanced solid tumors. J Clin Oncol 24: abstract 13012.
Hochster HS, Hart LL, Ramanathan RK, Hainsworth JD, Hedrick EE, Childs BH . (2006). Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): final analysis of the TREE-Study. J Clin Oncol 24: abstract 3510.
Holden SN, Ryan E, Kearns A, Holmgren E, Hurwtitz H . (2005). Benefit from bevazizumab (Bv) is independent of pre-treatment plasma vascular endothelial growth factor-A (pl-VEGF) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 23: abstract 3555.
Howdieshell TR, Callaway DBS, Webb WL, Gaines MD, Procter CD, Sathyanarayana et al. (2001). Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J Surg Res 96: 173–182.
Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM . (2004). Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res 64: 5355–5362.
Humblet Y, Peeters M, Bleiberg H, Stupp R, Sessa C, Roth A et al. (2005). An open-label, phase I study of cetuximab to assess the safety, efficacy and pharmacokinetics (PK) of different cetuximab regimens in patients with epidermal growth factor receptor (EGFR)-expressing metastatic colorectal cancer (mCRC). J Clin Oncol 23: abstract 3632.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342.
Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M et al. (2005). Association of k-ras, b-raf, and p-53 Status with the treatment effect of bevacizumab. J Natl Cancer Inst 97: 981–989.
Jennis A, Polikoff J, Mitchell E, Badarinath S, Graham C, Chen T et al. (2005). Erbitux (cetuximab) plus FOLFOX for colorectal cancer (EXPLORE): preliminary efficacy analysis of a randomized phase III trial. J Clin Oncol 23: abstract 3574.
Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS et al. (2006). Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24: 217–227.
Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G et al. (2003). Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21: 60–65.
Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht R et al. (2005). Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 23: 3697–3705.
Kemeny N, Garay CA, Gurtler J, Hochster H, Kennedy P, Benson A et al. (2004). Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol 22: 4753–4761.
Köhne C-H, Wils J, Lorenz M, Schoffski P, Voigtmann R, Bokemeyer C et al. (2003). Randomized phase III study of high-dose fluorouracil given as a weekly 24-h infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European Organization of Research and Treatment of Cancer Gastrointestinal Group study 40952. J Clin Oncol 21: 3721–3728.
Kozloff M, Hainsworth J, Badarinath S, Cohn A, Flynn P, Steis R et al. (2006). Efficacy of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRiTE). J Clin Oncol 24: abstract 3537.
Kretzschmar A, Cunningham D, Berry S, DiBartolomeo M, Michael M, Rivera F et al. (2006). Incidence of gastrointestinal perforations and bleeding in patients starting bevacizumab treatment in first-line mCRC without primary tumour resection: Preliminary results from the first BEATrial. ASCO GI: abstract 248.
Kubicka S, Arkenau H, Grothey A, Graeven U, Kretzschmar A, Greil R et al. (2006). Randomized trial of infusional 5-fluorouracil/folinic acid plus oxaliplatin (FUFOX) versus capecitabine plus oxaliplatin (CAPOX) as first line treatment of metastatic colorectal carcinoma (MCRC): final results of the safety and efficacy analysis. ASCO GI: abstract 277.
Labianca R, Floriani I, Cortesi E, Isa L, Zaniboni A, Marangolo M et al. (2006). Alternating versus continuous ‘FOLFIRI’ in advanced colorectal cancer (ACC): a randomized ‘GISCAD’ trial. J Clin Oncol 24: abstract 3505.
Lenz H-J, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P et al. (2006). Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol 24: 4914–4921.
Lim DH, Park YS, Park BB, Ji SH, Lee J, Park KW et al. (2005). Mitomycin-C and capecitabine as third-line chemotherapy in patients with advanced colorectal cancer: a phase II study. Cancer Chemother Pharmacol 56: 10–14.
Luo FR, Yang Z, Dong H, Camuso A, McGlinchey K, Fager K et al. (2005). Prediction of active drug plasma concentrations achieved in cancer patients by pharmacodynamic biomarkers identified from the geo human colon carcinoma xenograft model. Clin Cancer Res 11: 5559–5565.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al. (2004). Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139.
Lyseng-Williamson KA, Robinson DM . (2006). Bevacizumab. A review of its use in advanced colorectal cancer, breast cancer, and NSCLC. Am J Cancer 5: 43–60.
Maindrault-Goebel F, Lledo G, Chibaudel B, Mineur L, Andre T, Bennamoun M et al. (2006). OPTIMOX2, a large randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC). A GERCOR study. J Clin Oncol 24: abstract 3504.
Malik I, Hecht JR, Patnaik A, Venook A, Berlin J, Croghan G et al. (2005). Safety and efficacy of panitumumab monotherapy in patients with metastatic colorectal cancer. J Clin Oncol 23: abstract 3520.
Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J et al. (2004). Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res 10: 6487–6501.
Meropol NJ . (2005). Epidermal growth factor receptor inhibitors in colorectal cancer: it's time to get back on target. J Clin Oncol 23: 1791–1793.
Meyerhardt JA, Mayer RJ . (2005). Therapy for colorectal cancer. N Engl J Med 352: 476–487.
Miners AH, Garau M, Fidan D, Fischer AJ . (2005). Comparing estimates of cost effectiveness submitted to the National Institute for Clinical Excellence (NICE) by different organisations: retrospective study. BMJ 330: 365–368.
Morelli MP, Cascone T, Troiani T, Tuccillo C, Bianco R, Romano M et al. (2006). Antitumor activity of the combination of cetuximab, an anti-EGFR blocking monoclonal antibody and ZD6474, an inhibitor of VEGFR and EGFR tyrosine kinases. J Clin Oncol 24: abstract 13170.
Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F et al. (2005). Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol 6: 279–286.
Novotny WF, Holmgren E, Nelson B, Mass R, Kabbinavar F, Hurwitz H . (2004). Bevacizumab (a monoclonal antibody to vascular endothelial growth factor) does not increase the incidence of venous thromboembolism when added to first-line chemotherapy to treat metastatic colorectal cancer. J Clin Oncol 22: abstract 3529.
Nygren P, Sorbye H, Österlund P, Pfeiffer P . (2005). Targeted drugs in metastatic colorectal cancer with special emphasis on guidelines for the use of bevacizumab and cetuximab: an Acta Oncologica expert report. Acta Oncol 44: 203–217.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al. (2004). EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500.
Paramore LC, Thomas SK, Knopf KB, Cragin LS, Fraeman KH . (2006). Estimating costs of care for patients with newly diagnosed metastatic colorectal cancer. Clin Colorectal Cancer 6: 52–58.
Peeters M, Van Cutsem E, Siena S, Humblet Y, Hendlisz A, Neyns B et al. (2006). A phase 3, multicenter, randomized controlled trial of panitumumab plus best supportive care (BSC) vs BSC alone in patients with metastatic colorectal cancer. AACR: abstract CP-1.
Perez-Soler R, Zou Y, Li T, Tornos C, Lin Y . (2006). Topical vitamin K3 (Vit K3, Menadione) prevents erlotinib and cetuximab-induced EGFR inhibition in the skin. J Clin Oncol 24: abstract 3036.
Personeni N, Hendlisz A, Gallez J, Galdon MG, Larsimont D, Van Laethem JL et al. (2005). Correlation between the response to cetuximab alone or in combination with irinotecan and the activated/phosphorylated epidermal growth factor receptor in metastatic colorectal cancer. Semin Oncol 32: 59–62.
Pfeiffer P, Bjerregaard JK, Qvortrup C . (2007a). Biweekly cetuximab (Cet) and irinotecan (Iri) as third line therapy in patients with advanced colorectal cancer (ACRC). Proc ASCO GI: abstract 305.
Pfeiffer P, Nielsen D, Yilmaz M, Iversen A, Vejlø C, Jensen BV . (2007b). Cetuximab and irinotecan as third line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Acta Oncol: (in press).
Pfeiffer P, Sørbye H, Ehrsson H, Fokstuen T, Mortensen JP, Baltesgard L et al. (2006). Short-time infusion of oxaliplatin in combination with capecitabine (XELOX30) as second line therapy in patients with advanced colorectal cancer after failure to irinotecan and 5-fluorouracil. Ann Oncol 17: 252–258.
Pitot HC, Rowland KM, Sargent DJ, Philip PA, Mitchell EP, Goldberg RM et al. (2005). N9841: a randomized phase III equivalence trial of irinotecan (CPT-11) versus oxaliplatin/5-fluorouracil (5FU)/leucovorin (FOLFOX4) in patients (pts) with advanced colorectal cancer (CRC) previously treated with 5FU. J Clin Oncol 23: abstract 3506.
Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J et al. (2002). Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet 360: 671–677.
Ragnhammar P, Hafström L, Nygren P, Glimelius B . (2001). A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol 40: 282–308.
Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA . (2004). EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol 123: 982–989.
Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P et al. (2005). Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6: 491–500.
Rosati G, Rossi A, Germano D, Reggiardo G, Manzione L et al. (2003). Raltitrexed and mitomycin-C as third-line chemotherapy for colorectal cancer after combination regimens including 5-fluorouracil, irinotecan and oxaliplatin: a phase II study. Anticancer Res 23: 2981–2985.
Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK et al. (2003). Superiority of oxaliplatin and fluorouracil–leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil–leucovorin: interim results of a phase III trial. J Clin Oncol 21: 2059–2069.
Rougier P, Raoul JL, Van Laethem JL, Peeters M, Husseini F, Brezault C et al. (2004). Cetuximab+FOLFIRI as first-line treatment for metastatic colorectal CA. J Clin Oncol 23: abstract 3513.
Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R et al. (1998). Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352: 1407–1412.
Saif MW, Cohenuram M . (2006). Role of panitumumab in metastatic colorectal cancer. Clin Colorectal Cancer 6: 118–124.
Salomon DS, Brandt R, Ciardiello F, Normanno N . (1995). Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19: 183–232.
Saltz L, Rubin M, Hochster H, Tchekmeydian NS, Waksal H, Needle M et al. (2001). Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11 refractory colorectal cancer that expresses epidermal growth factor receptor. Proc Am Soc Clin Oncol 20: abstract 7.
Saltz LB, Chung KY, Timoney J, Park V, Hollywood E . (2006). Simplification of bevacizumab (bev) administration: do we need 90, 60, or even 30 min infusion times? J Clin Oncol 24: abstract 3542.
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ et al. (2000). Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Eng J Med 343: 905–914.
Saltz LB, Lenz H, Hochster H, Wadler S, Hoff P, Kemeny N et al. (2005). Randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetuximab/bevacizumab (CB) in irinotecan-refractory colorectal cancer. J Clin Oncol 23: abstract 3508.
Saltz LB, Meropol NJ, Loehrer Sr PJ, Needle MN, Kopit J, Mayer RJ . (2004). Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22: 1201–1208.
Saunders M, Iveson T . (2006). Management of advanced colorectal cancer: state of the art. Br J Cancer 95: 131–138.
Scappaticci FA, Fehrenbacher L, Cartwright T, Hainworth JD, Heim W, Berlin J et al. (2005). Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 91: 173–180.
Scartozzi M, Bearzi I, Berardi R, Mandolesi A, Fabris G, Cascinu S . (2004). Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol 22: 4772–4778.
Schrag D . (2004). The price tag on progress – chemotherapy for colorectal cancer. N Engl J Med 351: 317–319.
Schrag D, Chung KY, Flombaum C, Saltz L . (2005). Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst 97: 1221–1224.
Shia J, Klimstra DS, Li AR, Qin J, Saltz J, Teruya-Feldstein J et al. (2005). Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Mod Pathol 18: 1350–1356.
Skillings JR, Johnson DH, Miller K, Kabbinavar F, Bergsland E, Holmgren E et al. (2005). Arterial thromboembolic events (ATEs) in a pooled analysis of 5 randomized, controlled trials (RCTs) of bevacizumab (BV) with chemotherapy. J Clin Oncol 23: abstract 3019.
Sorbye H, Glimelius B, Berglund A, Fokstuen T, Tveit KM, Braendengen M et al. (2004). Multicentre phase II study of oxaliplatin combined with Nordic 5-fluorouracil/leucovorin bolus schedule (FLOX) as first-line treatment of metastatic colorectal cancer. J Clin Oncol 22: 31–38.
Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A et al. (2006). FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer 94: 798–805.
Starling N, Cunningham D . (2005). Second-line therapy for advanced colorectal carcinoma. Curr Oncol Rep 7: 173–180.
Stern M, Herrmann R . (2005). Overview of monoclonal antibodies in cancer therapy: present and promise. Crit Rev Oncol Hematol 54: 11–29.
Sugrue M, Kozloff M, Hainsworth J, Badarinath S, Cohn A, Flynn P et al. (2006). Risk factors for gastrointestinal perforations in patients with metastatic colorectal cancer receiving bevacizumab plus chemotherapy. J Clin Oncol 24: abstract 3535.
Tabernero J, Cervantes A, Martinelli E, Vega-Villegas E, Rojo A, Pérez-Fidalgo A et al. (2006). Optimal dose of cetuximab (C) given every 2 weeks (q2w): A phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of weekly (q1w) and q2w schedules in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 24: abstract 3085.
Tabernero JM, Van Cutsem E, Sastre J, Cervantes A, Van Laethem JL, Humblet Y et al. (2004). An international phase II study of cetuximab in combination with oxaliplatin/5-fluorouracil (5-FU)/folinic acid (FA) (FOLFOX-4) in the first-line treatment of patients with metastatic colorectal cancer (CRC) expressing Epidermal Growth Factor Receptor (EGFR). Preliminary results. J Clin Oncol 22: abstract 3512.
Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE et al. (2006). Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res 12: 2197–2207.
Tournigand C, Andr é T, Achille E, Lledo G, Flesh M, Mery-Mignard D et al. (2004). FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22: 229–237.
Tsuchihashi Z, Khambata-Ford S . (2005). Responsiveness to Cetuximab without mutations in EGFR. N Engl J Med 353: 208–209.
Vallbohmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA et al. (2005). Molecular determinants of cetuximab efficacy. J Clin Oncol 23: 3536–3544.
Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P et al. (2004). Oral capecitabine vs intravenous 5-fluorouracil and leucovorin; integrated efficacy data and novel analyses from two large, a randomised, phase III trials. Br J Cancer 90: 1190–1197.
Van Cutsem E, Humblet Y, Gelderblom H, Vermorken JB, Viret F, Glimelius B et al. (2007b). Cetuximab dose-escalation study in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on Cetuximab standard dose treatment (EVERST): Pharmacokinetic and efficacy data of a randomized study. Proc ASCO GI 2007: abstract 237.
Van Cutsem E, Michael M, Berry S, Dibartolomeo M, Rivera F, Kretzschmar A et al. (2007a). Preliminary safety and efficacy of bevacizumab with first-line FOLFOX, CAPOX, FOLFIRI, and capecitabine for mCRC: First BEATrial. Proc ASCO GI: abstract 346.
Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G et al. (2006). Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 42: 2212–2221.
Van Doorn R, Kirtschig G, Scheffer E, Stoof TJ, Giaccone G . (2002). Follicular and epidermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol 147: 598–601.
Venook A, Niedzwiecki D, Hollis D, Sutherland S, Goldberg R, Alberts S et al. (2006). Phase III study of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX)±cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results. J Clin Oncol 24 (185): abstract 3509.
Veronese ML, O'Dwyer PJ . (2004). Monoclonal antibodies in the treatment of colorectal cancer. Eur J Cancer 40: 1292–1301.
Vincenzi B, Santini D, Rabitti D, Coppola R, Beomonte Zobel B, Trodella L et al. (2006a). Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer 94: 792–797.
Vincenzi B, Santini D, Russo A, Silletta M, Gavasci M, Battistoni F et al. (2006b). Angiogenesis modifications related with cetuximab plus irinotecan as anticancer treatment in advanced colorectal cancer patients. Ann Oncol 17: 835–841.
Vincenzi B, Santini D, Tonini G . (2006c). Lack of response of cetuximab plus oxaliplatin in advanced colorectal cancer patients resistant to both oxaliplatin and cetuximab plus irinotecan. Ann Oncol 17: 527–528.
Wilke H, Glynne-Jones R, Thaler J, Adenis A, Preusser P, Aranda Aguilar E et al. (2006). MABEL – a large multinational study of cetuximab plus irinotecan in irinotecan resistant metastatic colorectal cancer. J Clin Oncol 24: abstract 3549.
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT et al. (2004). Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147.
Yamamoto DS, Viale PH, Zhao G . (2004). Severe acneiform rash. Clin J Oncol Nurs 8: 654–656.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfeiffer, P., Qvortrup, C. & Eriksen, J. Current role of antibody therapy in patients with metastatic colorectal cancer. Oncogene 26, 3661–3678 (2007). https://doi.org/10.1038/sj.onc.1210377
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210377
Keywords
This article is cited by
-
Effects of Chemotherapy for Metastatic Colorectal Cancer on the TGF-β Signaling and Related miRNAs hsa-miR-17-5p, hsa-miR-21-5p and hsa-miR-93-5p
Cell Biochemistry and Biophysics (2021)
-
Placebo-controlled phase II study of vitamin K3 cream for the treatment of cetuximab-induced rash
Supportive Care in Cancer (2017)
-
Dual inhibition of SRC and Aurora kinases induces postmitotic attachment defects and cell death
Oncogene (2012)
-
Anti-EGFR (cetuximab) combined with irinotecan for treatment of elderly patients with metastatic colorectal cancer (mCRC)
Journal of Cancer Research and Clinical Oncology (2012)
-
Combined treatment with Bevacizumab and standard chemotherapy restores abnormal immune parameters in advanced colorectal cancer patients
Investigational New Drugs (2012)