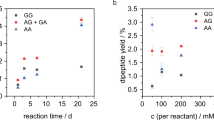
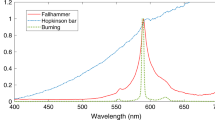
Abstract
BODENSTEIN1 studied this reaction in sealed bulbs containing excess of sulphur, and found a temperature coefficient of 1.34 between 234° and 283°, and 1.77 between 310° and 356°. His work was criticised, by Norrish and Rideal2, as possibly vitiated by the hydrogen sulphide liberated on the solidification of the residual sulphur, and as leaving uncertain, in view of the variable temperature coefficient, the part played by the walls of the bulbs. These workers employed a dynamic method, and showed that the logarithms of the total reaction velocity—always, however, measured in the presence of nitrogen—when plotted against absolute temperature yielded a curved line. The differences in the velocities obtained, using hydrogen at partial pressures of O.810 atm. and 0.304 atm., respectively, when suitably plotted gave a straight line which was interpreted as belonging to the gaseous reaction alone, since the surface reaction, common to each series, was assumed, and reasonably so, to be independent of pressure. The velocity of the composite reaction was also shown experimentally to be increased by increasing the glass surface, to which it was stated to be proportional; and to be independent of the amount of sulphur present. Knowing the contribution of the gaseous reaction, data for the surface were deduced, and thus the temperature coefficient of each reaction was obtained: homogeneous 2.19, heterogeneous 1.48.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Z. phys. Chem., 29, 315; 1899.
J. Chem. Soc., 123, 696; 1923.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
AYNSLEY, E., PEARSON, T. & ROBINSON, P. New Experimental Evidence in the Sulphur-Hydrogen Reaction. Nature 131, 471–472 (1933). https://doi.org/10.1038/131471a0
Issue Date:
DOI: https://doi.org/10.1038/131471a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.