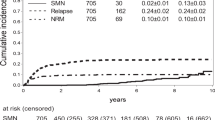
Summary:
The incidence of secondary myelodysplasia/acute myeloid leukemia (AML) was retrospectively assessed in an international joint study in 305 node-positive breast cancer patients, who received mitoxantrone-based high-dose chemotherapy (HDCT) followed by autologous stem cell support as adjuvant therapy. The median age of the patients was 57 years (range 22–67). In all, 268 patients received peripheral blood stem cells, and 47 patients received autologous bone marrow. After a median follow-up of 57 months (range 10–125), three cases of secondary AML (sAML) were observed, resulting in a cumulative incidence of 0.94%. One case of sAML developed 18 months after HDCT (FAB M3) The karyotype was translocation 15;17 and, after induction therapy, the patient underwent autologous stem cell transplantation, and is in complete remission (CR) of both breast cancer and AML. The second patient developed AML (FAB M4eo with inversion 16) 5 months after HDCT. This patient achieved CR after induction therapy, but died of infectious complication. A third patient developed AML (FAB M4) 6 months after HDCT. She achieved CR after induction therapy, but relapsed and expired 28 months after diagnosis of AML. sAML after mitoxantrone-based HDCT is a possible, but rare complication in breast cancer patients.
Similar content being viewed by others
Main
Secondary myelodysplasia (MDS) or acute myeloid leukemia (AML) in breast cancer patients is a rare but well-known long-term complication of prior chemotherapy or radiation therapy as adjuvant therapy.1,2,3 Specific risk factors are the combinations of chemotherapy and radiation therapy, the cumulative dose of alkylating agents and the duration of therapy.2 Two different types of treatment-related leukemia can be distinguished. The first type results from prior therapy with alkylating agents or radiation therapy, and occurs after a latency period of 5–7 years. This type of AML is often preceded by a preleukemic period of MDS. Nearly 90% of the patients with alkylating agent-related MDS or AML show clonal chromosome aberrations including monosomy or deletions on chromosomes 5 and/or 7 or complex aberrations involving chromosomes 3, 12, 17, and 21.4 The second type of therapy-related leukemia is induced by topoisomerase II-targeted drugs like etoposide, anthracyclines or, recently, anthracenediones.5,6,7 This type of AML usually occurs after a median of 2 years and is not preceded by a myelodysplastic syndrome. According to the French–American–British (FAB) classification, more frequently, M4 or M5 subtype is observed and cytogenetic analysis showed a high frequency of rearrangements of the chromosome band as 11q23, translocation (t)(8;21), t(15;17), inversion 16 (inv(16)) or t(8;16), as in de novo AML.5,8 Recently, several studies indicated that adjuvant chemotherapy consisting of the anthracenedione mitoxantrone, a topoisomerase II-targeted drug, may induce secondary AML (sAML) in up to 8% of patients7,9,10,11,12,13,14,15 (see Table 1). Few studies investigated the risk of secondary MDS/AML after high-dose chemotherapy (HDCT) in breast cancer patients, suggesting a similar incidence as conventional chemotherapy.16,17,18,19 Mitoxantrone is part of several high-dose chemotherapy regimens followed by autologous stem cell transplantation in breast cancer patients.20,21,22,23 In the present study, we evaluate the incidence of secondary MDS/AML in 305 breast cancer patients after mitoxantrone-based high-dose conditioning regimens, followed by autologous stem cell transplantation in a joint analysis of the Solid Tumor Working Party of the European Group for Blood and Marrow Transplantation (EBMT), the German Adjuvant Breast Cancer Study Group (GABG), and the University of California, San Francisco (UCSF).
Materials and methods
Patient population and data collection
In all, 305 patients with node-positive breast cancer had received mitoxantrone-based HDCT followed by auto-logous stem cell transplantation as adjuvant therapy between July 1990 and December 1998. All centers of the EBMT received a questionnaire for the transplanted patients. The patients of the GABG and the UCSF were treated in phase II or III protocols and monitored closely. A total of 305 patients were evaluable to calculate the incidence of secondary myelodysplastic syndromes or leukemias. All patients were high-risk patients with at least more than three involved lymph nodes, either stage II/III or IIIB. The different conditioning regimens are listed in Table 2. Most of the patients received four to six cycles of anthracycline-based induction chemotherapy prior to HDCT. Patients with stage IIIB generally received in the San Francisco study two to four premastectomy cycles of chemotherapy, followed by one to two more cycles prior to HDCT. In all, 251 patients received a locoregional radiotherapy of the chest wall or breast including the regional lymph nodes (supraclavicular and axillary), but detailed analysis of radiotherapy was not available in all patients. In 54 patients, no data of radiotherapy were available. Usually patients with positive hormonal receptor status received 20–30 mg tamoxifen for a planned 5 years post HDCT. The median age of the patients at the time of stem cell transplantation was 46 years (range 22–67). The stem cell source was bone marrow in 47 patients and peripheral blood stem cells in 258 patients.
Statistical analysis
The time to MDS/AML is the interval from HDCT to diagnosis of MDS/AML. Patients who did not develop MDS/AML were censored at the date of death or at the date of last follow-up for surviving patients. The time to MDS/AML and the cumulative probability of developing MDS/AML was estimated by the Kaplan–Meier method.
Results
After a median follow-up of 57 months (range 10–125), three out of 305 patients developed secondary leukemia in this retrospective study. Thus, the cumulative incidence of secondary leukemia is 0.94%. All patients developed AML without preceding MDS within 2 years after HDCT. The Kaplan–Meier probability of developing MDS at 2 (242 at risk), 4 (145 at risk), 6 (49 at risk), and 8 years (19 at risk) was 1% (95% CI: 0.094–1.06%). One of the three cases of sAML developed in a 31-year-old woman with inflammatory breast cancer 18 months after HDCT (FAB M3) The karyotype was t(15;17) and, after induction therapy with daunorubicine and ATRA and consolidation with high-dose cytosine arabinoside, the patient underwent autologous stem cell transplantation after conditioning with busulfan (16 mg/kg) and etoposide (40 mg/kg). The patient is still in complete remission (CR) of both breast cancer and AML 63 months after stem cell transplant for breast cancer and 42 after stem cell transplant for AML. The second 52-year-old patient with more than 10 involved lymph nodes received HDCT after 4 cycles of induction chemotherapy with epirubicine and cyclophosphamide. sAML (FAB M4eo with inv 16) developed 5 months after HDCT. This patient received anthracycline-based induction therapy and consolidation therapy with high-dose cytosine-arabinoside and achieved CR, but died of infectious complications. A third 60-year-old woman developed AML (FAB M4) 6 months after HDCT. She achieved CR after ARA-C and daunorubicin therapy, but relapsed and expired 28 months after diagnosis of AML. All patients had received peripheral blood progenitor cells as the stem cell source for HDCT (Table 3).
Discussion
Mitoxantrone is an anthracenedione derivate that intercalates into DNA and RNA. It damages DNA by interacting with topoisomerase II. Like anthracyclines, it arrests the cell cycle in G2 and S phases.25 As part of a combination chemotherapy or alone, it is used as an active agent in the treatment of breast cancer patients. Recently, several reports have shown an increased incidence of secondary MDS/AML up to 8% after adjuvant chemo-therapy containing the anthracenedione derivate mitoxantrone.7,9,10,11,12,13,14,24 Since mitoxantrone was mainly used as part of a combination chemotherapy followed by radiation therapy, the responsibility of the combination chemotherapy in the genesis of treatment-related AML cannot be excluded. In a French population-based study with 3093 women, the risk of leukemia was significantly increased in women who received adjuvant mitoxantrone-based chemo-therapy and radiotherapy, with a standardized incidence ratio of 28.5. In that study, a dose-dependent effect was seen for mitoxantrone and the incidence of leukemia was lower in patients treated with anthracyclines than in those treated with mitoxantrone >13 mg/m2.15
In a smaller study, a standardized incidence ratio of 38 for breast cancer patients treated adjuvant with a combination of mitoxantrone, 5-fluorouracil, and cyclophosphamide was reported.10 The high incidence of AML with an incidence ratio of 339 in the study by Andersson et al24 was ascribed to prednimustine, a known leukemogenic alkylating agent, but a synergistic or additive effect of mitoxantrone cannot be excluded. Regarding this risk of developing sAML after standard mitoxantrone treatment, one might expect a higher incidence after a mitoxantrone-based HDCT regimen. In our study, we observed two cases of sAML, which occurred as described for topoisomerase II-induced leukemias within the first 2 years after treatment and without preceding MDS. The karyotype abnormalities found in these two patients were t(15;17) and inv(16). Besides the balanced translocation 11q23, inv(16), and t(15;17) are found with a high frequency in topoisomerase II-induced sAML, especially after treatment with mitoxantrone.7,8,26 In a French study, two out of 10 patients with sAML after mitoxantrone-based chemotherapy for breast cancer showed an inv(16), and one patient the translocation t(15;17).7 Furthermore, Carli et al found in four out of nine patients (44%) with sAML after mitoxantrone-based chemotherapy for breast cancer, a FAB M3 subtype with classical translocation t(15;17).26
After autologous transplantation for non-Hodgkin lymphoma, there is some concern about the high probability of secondary MDS or AML, which is 14–18% at 5 years.27,28,29 There are only a few reports of MDS/AML following HDCT for breast cancer. In 864 patients who received a high-dose regimen consisting of BCNU, cyclophosphamide, and cisplatinum, a 4-year probability of developing MDS/AML of 1.6% has been reported.17 In a Spanish trial involving 229 patients after a median follow-up of 36 months, no single case of secondary MDS/AML was observed.19 In that trial, cytogenetic aberrations were found in some patients (5%), after HDCT, but these aberrations were only transient and disappeared without developing into MDS or AML. Recently, in a retrospective EBMT study, only one case of treatment-related AML with 11q23 translocation was observed in 364 patients with primary breast cancer after HDCT.16 Another retrospective analysis including 379 patients with breast cancer after HDCT reported a probability at 5 years for developing a secondary MDS/AML of 0.032%.30
While, in general, the prognosis of sAML occurring after topoisomerase II inhibitor treatment is poor,31 there are controversial reports about the prognosis in patients with balanced translocations t(8;21), t(15;17) or inv(16) after topoisomerase II inhibitor treatment. Some investigators reported these cytogenetic abnormalities as favorable with the same response, and survival as de novo AML,32,33 but others reported a poor outcome.7,31,34 One patient in our study with AML M3 t(15;17) received a second autologous transplant and is now more than 3 years disease-free from both breast cancer and AML. The outcome of therapy-related acute promyelocytic leukemia (APL) has been recently reported to be similar to de novo APL.35 The other patient with AML M4eo inv(16) achieved CR, but died during treatment of infectious complications. Only the death of the third patient was due to relapse of AML. In our study, no case of MDS has been observed. However, because no cytogenetic monitoring was performed, anemia or thrombocytopenia occurred, so that overlooking of MDS cannot be excluded from this retrospective study.
We conclude that anthracendione-based high-dose treatment with mitoxantrone in combination with cyclophos-phamide±thiotepa and local radiation therapy induces sAML in some rare cases. The incidence of sAML is lower in comparison to standard mitoxantrone doses used as adjuvant therapy. A single high dose of mitoxantrone might not be as leukemogenic as smaller doses given at fixed intervals. Since all patients also received anthracycline-based induction chemotherapy, the contribution of anthracyclines to the development of sAML cannot be excluded. Since, in our patients, no MDS was preceded and two of them showed t(15;17) and inv(16), respectively, the development of AML is likely attributed to the topoiso-merase II inhibitors than to the alkylating agents used in the HDCTregimen. The low incidence of about 1% is in contrast to the reported high incidence after autologous transplantation for non-Hodgkin's lymphoma. There is no obvious increase in leukemia in comparison to the standard dose of mitoxantrone, but longer follow-up is needed to determine the late incidence of AML.
References
Curtis RE, Boice JD, Moloney WC et al. Leukemia following chemotherapy for breast cancer. Cancer Res 1990; 50: 2741–2746.
Curtis RE, Boice Jr JD, Stovall M et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med 1992; 326: 1745–1751.
Fisher B, Rockette H, Fisher ER et al. Leukemia in breast cancer patients following adjuvant chemotherapy or postoperative radiation: the NSABP experience. J Clin Oncol 1985; 12: 1640–1658.
LeBeau MM, Albain KS, Larson RA et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no 5 and 7. J Clin Oncol 1986; 4: 325–345.
Felix CA . Secondary leukemias induced by topoisomerase-targeted drugs. Biochem Biophys Acta 1998; 1400: 233–255.
Kollmannsberger C, Hartmann JT, Kanz L et al. Risk of secondary myeloid leukemia and myelodysplastic syndrome following standard-dose chemotherapy or high-dose chemo-therapy with stem cell support in patients with potentially curable malignancies. J Cancer Res Clin Oncol 1998; 124: 207–214.
Linassier C, Barin C, Calais G et al. Early secondary acute myelogenous leukemia in breast cancer patients after treatment with mitoxantrone, cyclophosphamide, fluorouracil and radiation therapy. Ann Oncol 2000; 11: 1289–1294.
Pedersen-Bjergaard J, Andersen MK, Johansson B . Balanced chromosome aberrations in leukemias following chemotherapy with DNA-topoisomerase II inhibitors. J Clin Oncol 1998; 16: 1897–1898.
Saso R, Kulkarni S, Mitchell P et al. Secondary myelodysplastic syndrome/acute myeloid leukaemia following mitoxantrone-based therapy for breast carcinoma. Br J Cancer 2000; 83: 91–94.
Kumpulainen EJ, Hirvikovski PP, Pukkula E et al. Cancer risk after adjuvant chemo- or chemohormonal therapy for breast cancer. Anticancer Drug 1998; 9: 131–134.
Mellilo LMA, Sajeva MER, Musto P et al. Acute myeloid leukemia following 3M (mitoxantrone, mitomycin, methotrexate) chemotherapy for advanced breast cancer. Leukemia 1997; 1: 2211–2212, (letter).
Mitchell PLR, Treleaven J, Swansbury GJ et al. Secondary acute myeloid leukemia (AML) and myelodysplasia (MDS) following mitoxantrone given as adjuvant therapy for breast cancer. Proc Am Soc Clin Oncol 1996; 15: 127a (abstract 173).
Philpott NJ, Bevan DH, Gordon Smith EC . Secondary leukemia after MMM combined modality therapy for breast carcinoma. Lancet 1993; 341: 1289–1290, (letter).
Cremin P, Flattery M, McCann SR et al. Myelodysplasia and acute myeloid leukaemia following adjuvant chemotherapy for breast cancer using mitoxantrone and methotrexate with or without mitomycin. Ann Oncol 1996; 7: 745–746.
Chaplain G, Milan C, Sgro C et al. Increased risk of acute leukemia after adjuvant chemotherapy for breast cancer: a population-based study. J Clin Oncol 2000; 18: 2836–2842.
Kröger N, Zander AR, Martinelli G et al. Low incidence of secondary myelodysplasia (sMDS) and acute myeloid leukaemia (sAML) after high-dose chemotherapy as adjuvant therapy for breast cancer patients: a study of the Solid Tumors Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol 2003; 14: 554–558.
Laughlin MJ, McGaughey DS, Crews JR et al. Secondary myelodysplasia and acute leukemia in breast cancer patients after autologous bone marrow transplant. J Clin Oncol 1998; 16: 1008–1012.
Roman-Unfer S, Bitran JD, Hanauer S et al. Acute myeloid leukemia and myelodysplasia following intensive chemotherapy for breast cancer. Bone Marrow Transplant 1995; 16: 163–168.
Martinez-Climent JA, Comes AM, Vizcarra E et al. Chromosomal abnormalities in women with breast cancer after autologous stem cell transplantation are infrequent and may not predict development of therapy-related leukemia or myelodysplastic syndrome. Bone Marrow Transplant 2000; 25: 1203–1208.
Damon LE, Wolf JL, Rugo HS et al. High-dose chemotherapy (CTM) for breast cancer. Bone Marrow Transplant 2000; 26: 257–268.
Gisselbrecht C, Extra JM, Lotz JP et al. Cyclophosphamide/mitoxantrone/melphalan (CMA) regimen prior to autologous bone marrow transplantation (ABMT) in metastatic breast cancer. Bone Marrow Transplant 1996; 18: 857–863.
Wallerstein Jr R, Spitzer G, Dunphy F et al. A phase II study of mitoxantrone, etoposide, and thiotepa with autologous bone marrow support for patients with relapsed breast cancer. J Clin Oncol 1990; 8: 1782–1788.
Zander AR, Krüger W, Kröger N et al. High dose mitoxantrone with thiotepa, cyclophosphamide and autologous stem cell rescue for high risk stage II and stage III breast cancer. Bone Marrow Transplant 1996; 18 (Suppl 1): 24–25.
Anderson M, Philip P, Pedersen-Bjergaard J . High risk of therapy-related leukemia and preleukemia after therapy with prednimustine, methotrexate, 5-fluorouracil, mitoxantrone and tamoxifen for advanced breast cancer. Cancer 1990; 65: 460–464.
Bowden GT, Roberts R, Alberts DS et al. Comparative molecular pharmacology in leukemic L1210 cells of the anthracene anticancer drugs mitoxantrone and bisantrene. Cancer Res 1985; 45: 4915–4920.
Carli PM, Sgro C, Parchin-Geneste N et al. Increased therapy-related leukemia secondary to breast cancer. Leukemia 2000; 14: 1014–1017.
Darrington DL, Vose JM, Anderson JR et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol 1994; 12: 2527–2534.
Stone RM, Neuberg D, Soiffer R et al. Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 1994; 12: 2535–2542.
Milligan DW, Ruiz De Elvira MC, Kolb HJ et al. Secondary leukemia and myelodysplasia after autografting for lymphoma: results from the EBMT. Br J Haematol 1999; 106: 1020–1026.
Nichols G, de Castro K, Wei LX et al. Therapy-related myelodysplastic syndrome after autologous stem cell transplantation for breast cancer. Leukemia 2002; 16: 1673–1679.
Pui CH, Relling MV, Rivera GK et al. Epipodophyllotoxin-related acute myeloid leukemia: a study of 35 cases. Leukemia 1995; 9: 1990–1996.
Kantarjian HM, Keating MJ . Therapy-related leukemia and myelodysplastic syndrome. Semin Oncol 1987; 14: 435–438.
Quesnel B, Kantarjian H, Bjergaard J et al. Therapy-related acute myeloid leukemia with t (8;21), inv(16), and t (8;16): a report on 25 cases and review of the literature. J Clin Oncol 1993; 11: 2370–2379.
Hoffmann L, Moller P, Pedersen-Bjergaard J et al. Therapy-related acute promyelocytic leukemia with t (15;17) (q22;q12) following chemotherapy with drugs targeting DNA topoisomerase II. A report of two cases and review of the literature. Ann Oncol 1995; 6: 781–788.
Beaumont M, Sanz M, Carli PM et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol 2003; 21: 2123–2137.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Kröger, N., Damon, L., Zander, A. et al. Secondary acute leukemia following mitoxantrone-based high-dose chemotherapy for primary breast cancer patients. Bone Marrow Transplant 32, 1153–1157 (2003). https://doi.org/10.1038/sj.bmt.1704291
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704291
Keywords
This article is cited by
-
Current treatment strategies in relapsed/refractory mantle cell lymphoma: where are we now?
International Journal of Hematology (2017)
-
A review of infectious complications after haploidentical hematopoietic stem cell transplantations
Infection (2017)
-
Does autologous transplantation directly increase the risk of secondary leukemia in lymphoma patients?
Bone Marrow Transplantation (2007)