Abstract
The Wnt proteins constitute a large family of extracellular signalling molecules that are found throughout the animal kingdom and are important for a wide variety of normal and pathological developmental processes1,2. Here we describe Wnt-inhibitory factor-1 (WIF-1), a secreted protein that binds to Wnt proteins and inhibits their activities. WIF-1 is present in fish, amphibia and mammals, and is expressed during Xenopus and zebrafish development in a complex pattern that includes paraxial presomitic mesoderm, notochord, branchial arches and neural crest derivatives. We use Xenopus embryos to show that WIF-1 overexpression affects somitogenesis (the generation of trunk mesoderm segments), in agreement with its normal expression in paraxial mesoderm. In vitro, WIF-1 binds to Drosophila Wingless and Xenopus Wnt8 produced by Drosophila S2 cells. Together with earlier results obtained with the secreted Frizzled-related proteins1,2, our results indicate that Wnt proteins interact with structurally diverse extracellular inhibitors, presumably to fine-tune the spatial and temporal patterns of Wnt activity.
Similar content being viewed by others
Main
There are two families of secreted molecules known to inhibit Wnt signalling activity: the secreted Frizzled-related protein (sFRP) family, whose members all have an amino-terminal cysteine-rich domain (CRD) that is highly homologous to the ligand-binding domain of Frizzled proteins, which are transmembrane Wnt receptors1,2; and the protein family Dickkopf (Dkk), whose mechanism of action is at present unknown3.
WIF-1 was first identified as an expressed sequence tag from the human retina (J. P. Macke, P.M.S. and J.N., unpublished results), and highly conserved orthologues have been isolated from mouse, Xenopus and zebrafish (Fig. 1). The deduced amino-acid sequence of WIF-1 has an N-terminal signal sequence, a domain of ∼150 amino acids (the WIF domain), five epidermal growth factor (EGF)-like repeats that are most similar to those of the extracellular matrix protein tenascin, and a hydrophilic domain of ∼45 amino acids at the carboxy terminus.
Top, alignment of the WIF-1 proteins from human (h), mouse (m), Xenopus (x), and zebrafish (z). Dashes indicate amino-acid identity and dots indicate a gap. The locations of the five epidermal growth factor (EGF) repeats are indicated by lines above the sequence. Bottom, domain structure of WIF-1, showing, from N to C terminus: SS, signal sequence; WIF domain; five EGF repeats; and a hydrophilic C terminus.
In the adult mouse, WIF-1 expression is highest in the heart and lung, and lower in the brain and eye (Fig. 2a). Northern-blot hybridization with Xenopus total RNA revealed the presence of a single transcript expressed first at neurula stages (not shown), and in situ hybridization to Xenopus or zebrafish embryos confirmed that no messenger RNA is detectable at the gastrula stage. In Xenopus, WIF-1 is expressed during somitogenesis predominantly in the unsegmented paraxial presomitic mesoderm and to a much lesser extent in newly segmented somites (Fig. 2b–e). In zebrafish, WIF-1 is highly expressed in unsegmented paraxial mesoderm and is virtually undetectable in mature somites (Fig. 2g–i). WIF-1 expression is visible in both species in the notochord in register with mature somites, but not with unsegmented paraxial mesoderm (Fig. 2d–i). WIF-1 mRNA is also present in discrete domains of the anterior brain (Fig. 2f, j), as well as in the visceral arches, nasal placodes and otic vesicles (Fig. 2e, f).
a, RNase protection assay using a mWIF-1 probe (top) or a mouse RNA polymerase II large-subunit probe (bottom); B, brain; E, eye; H; heart; K, kidney; Li, liver; Lu, lung; S, spleen; T, testis; Y, yeast tRNA. b–j, WIF-1 mRNAs detected by in situ hybridization. b–f, Xenopus; g–j, zebrafish. Expression is first detectable at the onset of somitogenesis in paraxial mesoderm (b, g). Expression is prominent in unsegmented presomitic mesoderm (psm), and is much weaker in newly formed somites (som; c, d, h, i). WIF-1 is expressed in the notochord (not) of 15-somite-stage zebrafish embryos (h) and stage-24 Xenopus embryos (d) in register with mature somites; no expression is detected in notochord regions flanked by unsegmented paraxial mesoderm. This pattern persists until the late stages of somitogenesis (e, i). In Xenopus tadpoles, strong WIF-1 expression is seen in visceral arches (va), and also in otic vesicles (ov), nasal placodes (npl) and several regions of the anterior brain (f). In the 24-h zebrafish embryo, expression is seen in the epiphysis (ep) and ventral midbrain (vmb) (j).
To investigate the activity of WIF-1 in vivo, we injected early Xenopus embryos with synthetic RNA encoding human WIF-1 (hWIF-1) either alone or in combination with developmental regulators. Overexpression of hWIF-1 provoked anteriorization and dorsalization of the embryos, as revealed by the formation of secondary body axes upon ventral injection and by the enlargement of antero–dorsal structures upon dorsal injection (Fig. 3a–f). Such phenotypes are also seen in embryos in which the late ventralizing XWnt8 pathway has been blocked by a dominant-negative Wnt ligand or by overexpression of the secreted Wnt inhibitor sFRP-3/Frzb (refs 1,2,4). By using Wnt-dependent axis induction in early Xenopus embryos as an assay, we found that hWIF-1 blocks the activity of XWnt8 in a dose-dependent manner (Table 1). In this assay, the N-terminal WIF domain (WD) of hWIF-1 is as effective as full-length hWIF-1. Inhibition also occurs following injection of XWnt8 and hWIF-1 separately in adjacent blastomeres, suggesting that the interaction occurs in the extracellular space (Table 1). In a second measure of XWnt8 inhibition, the late ventralizing activity of XWnt8, generated by overexpression from DNA expression constructs at gastrulation5,6, was also antagonized by co-injection of DNA encoding hWIF-1 (not shown). The inhibition is specific to Wnt ligands because axis induction by the transcription factor Siamois, which is activated by the Wnt pathway, is not inhibited, nor is the secreted dorsalizing agent Chordin (Table 1).
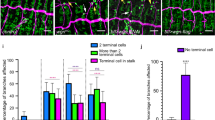
a–f, WIF-1 dorsalizes Xenopus embryos. hWIF-1 RNA (500 pg per blastomere) was injected into dorsal blastomeres (a, c, e) or ventral blastomeres (b, f) at the 4-cell stage. Dorsal injections led to anteriorization and hyperdorsalization of the embryos, as revealed by the formation of an enlarged head at the tadpole stage (a), an expanded cement gland (cg) field at the neurula stage (c), and an enlarged notochord visible in sections (e; control embryo in d). Ventral injections led to the formation of a partial secondary axis in 20% of the embryos (b) containing muscle (mus) and neural tissue (nt) (f). g–i, WIF-1 synergizes with chordin to generate a complete secondary axis (Table 1). g, Co-injection of chordin and Xenopus sFRP-3/Frzb RNAs gave rise to embryos with a complete but cyclopic secondary axis. h, By contrast, co-injection of chordin and hWIF-1 gave rise to embryos with a complete secondary axis; two separated eyes are evident. i, Co-injection of chordin and WD RNA does not promote two-eyed ectopic heads. j–p, WIF-1 affects the process of somite formation. 4-cell embryos were injected on one side with 100 pg pCS2+/hWIF-1 DNA and β-galactosidase RNA. β-Galactosidase activity is seen as brown dots and embryos were probed for XMyoD (j–n). The posterior domain of XMyoD expression in presomitic mesoderm was suppressed by hWIF-1 injection at the late neurula stage (j, dorsal view; k, posterior view). Segmentation is impaired by hWIF-1 injection, as revealed by XMyoD expression in mature somites of a stage-20 embryo (l) and a stage-26 embryo (m, n), and by a Hoechst-stained horizontal section of a stage-26 embryo (o, p). Note the disorganization of somites with occasional fusions (arrows in o). The process of somite rotation (rot) is delayed and myotome nuclei do not align properly (p).
hWIF-1 inhibits XWnt8 more than Wingless (Wg), and XWnt3A is only weakly inhibited (Table 1). These results place WIF-1 in thesame category as sFRP-3/Frzb, as a putative secreted Wnt antagonist. However, the activities of these two factors differ when measured by co-injection with the BMP inhibitor Chordin. Co-injection of Chordin with sFRP-3/Frzb promoted complete secondary axis formation at low frequency and ectopic heads were always cyclopic, as reported earlier3 (Fig. 3g; Table 1), whereas co-injection of Chordin and hWIF-1 gave complete secondary axes in all cases, with most having two eyes (Fig. 3h; Table 1). This difference is probably qualitative rather than quantitative as we were unable to obtain two-eyed secondary heads with Chordin and higher doses of sFRP-3/Frzb. The WIF domain alone could also synergize with Chordin to induce ectopic heads, but it did not promote separation of the eyes (Fig. 3i; Table 1), suggesting that the EGF-like repeats are necessary for full activity of WIF-1.
As WIF-1 is expressed in paraxial mesoderm in both zebrafish and Xenopus, we tested the effect of WIF-1 overexpression on somite formation by injecting one side of Xenopus 4-cell embryos with hWIF-1 DNA to initiate expression by the onset of gastrulation: we found that hWIF-1 specifically antagonized the formation of the most posterior presomitic mesoderm (Fig. 3j, k). At later stages of development, hWIF-1-injected embryos consistently had abnormally shaped and disorganized somites in which the process of somite rotation was impaired on the injected side and was occasionally accompanied by fusions of adjacent somites (Fig. 3l–p). We suggest that WIF-1 is involved in segmentation of the mesoderm, perhaps by antagonizing an as-yet unidentified Wnt molecule.
To assess the inhibitory effect of WIF-1 on Wnt signalling in a simplified system, we determined the effect of hWIF-1, an IgG heavy-chain fusion containing full-length hWIF-1 (WIF-1–IgG), or the IgG heavy chain alone (IgG) on Wg-dependent Armadillo stabilization in Drosophila clone-8 cells (ref. 7; Fig. 4a, b). At 30 nM, WIF-1–IgG or hWIF-1 significantly inhibit the response of clone-8 cells to Wg-conditioned medium. To determine the mechanism of WIF-1 inhibition, we tested whether WIF-1 could bind to soluble, secreted Wnt proteins. To produce a functional secreted vertebrate Wnt, we transfected S2 cells with a complementary DNA encoding XWnt8 tagged at its C terminus with a Myc epitope (XWnt8–Myc). The resulting conditioned medium contained soluble XWnt8–Myc, which stabilizes Armadillo in Drosophila clone-8 cells and β-catenin in C57MG cells, and can bind to the surface of 293 cells transfected with any one of several members of the Frizzled family (J.-C.H., A.R., P.M.S. and J.N., unpublished results). This XWnt8–Myc-conditioned medium, an analogous conditioned medium containing Wg, and a control conditioned medium from untransfected S2 cells were used in the following experiments.
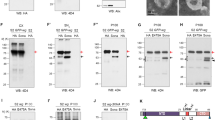
a, Characterization of hWIF-1 and WIF-1–IgG fusion proteins secreted from transiently transfected 293 cells by immunoblotting with anti-human IgG (left) or affinity-purified anti-WIF-domain antibodies (right). Each lane contains 40 µl 20-fold concentrated serum-free conditioned medium. b,hWIF-1 inhibits Wg signalling by clone-8 cells. Clone-8 cells were treated with control (lane 1) or Wg-containing (lanes 2–6) medium from S2 cells that had been preincubated with DMEM/F-12 (lanes 1, 2) or with 0.4 ml control medium (lane 3) or medium containing 30 nM WIF-1–IgG (lane 4), IgG (lane 5), or WIF-1 (lane 6). Similar results were obtained in 6 independent experiments. c, d, WIF-1–IgG and WD–IgG bind Wg (c) and XWnt8-Myc (d). Lane 1, 40 µl control S2 medium; lane 2, 10% of the Wg or XWnt8-Myc medium used in each binding reaction; lanes 3–5, 30% (c) or 40% (d) of the bound material; lanes 6–8, 10% of the unbound fraction from the samples in lanes 3–5, respectively. e, Reversible binding of WIF-1–IgG to Wg (top) and XWnt8–Myc (bottom). In lanes 1 and 2, 10 pmol WIF-1–IgG was prebound to protein A-Sepharose, and then added to 250 µl Wg (top) or 200 µl XWnt8–Myc (bottom) medium that had been preincubated for 2 h at 4 °C with control medium (lane 1) or medium containing 200 pmol hWIF1. After 2 h incubation at 4 °C, the beads were processed as described in Methods. In lanes 3 and 4, 10 pmol WIF-1–IgG prebound to protein A–Sepharose was precincubated with 250 µl Wg (top) or 200 µl XWnt8–Myc (bottom) medium for 2 h at 4 °C, and the unbound proteins removed with five washes of PBS. Control medium (lane 3) or medium containing 200 pmol hWIF-1 was added to the beads, which were then incubated for 2 h at 4 °C and washed as for lanes 1 and 2. f, hWIF-1 competes efficiently with both WIF-1–IgG and Dfz2CRD–IgG for XWnt8–Myc binding, but msFRP-3 competes efficiently only with Dfz2CRD. 100 µl XWnt8–Myc medium was incubated at 4 °C for 1 h with 100 µl control medium (lanes 1, 4), 100 µl medium containing 200 pmol hWIF-1 (lanes 2–5) and 200 pmol purified SFRP-3 (lanes 3–6), 5 pmol WIF-1-IgG (lanes 1–3) or Dfz2CRD–IgG (lanes 4–6) were incubated with protein A–Sepharose, and the washed beads were then added to each sample for an additional 2 h incubation at 4 °C. The XWnt8–Myc/WIF-1–IgG complex is resistant, whereas the XWnt8–Myc/Dfz2CRD–IgG complex is susceptible to msFRP-3 competition. Both complexes are susceptible to competition by hWIF1. g, Left, binding of XWnt8–AP to WIF-1 (squares, dashed line), WIF-1–IgG (circles, solid line) and IgG (filled circles, solid line). The vertical axis shows the increase in absorbance at 405 nm following cleavage of p -nitrophenylphosphate. Right, Scatchard plot of the data for XWnt8–AP binding to WIF-1–IgG. The data for XWnt8–AP binding to WIF-1 give an almost identical best-fit line (not shown). Vertical axis, ratio of bound to free XWnt8–AP; horizontal axis, concentration of bound XWnt8–AP. The fit of the data to a binary binding model gave k D values of 16 and 18 nM for WIF-1 and WIF-1–IgG, respectively.
WIF-1–IgG and an IgG fusion containing only the WIF domain (WD-IgG) co-precipitated Wg and XWnt8–Myc, with XWnt8–Myc being more efficient (Fig. 4c, d). The moderately efficient precipitation of Wg by hWIF-1–IgG (∼10% co-precipitation; Fig. 4c) contrasts to the efficient inhibition by hWIF-1 and WIF-1–IgG of the Armadillo response to added Wg in clone-8 cells (Fig. 4b). This apparent discrepancy is explained if only a fraction of secreted Wg protein is active and can bind hWIF-1, WIF-1–IgG and the receptor on clone-8 cells. Binding seems to be specific and at least partially reversible, because a 20-fold excess of hWIF-1 can compete efficiently with WIF-1–IgG for binding to Wg or to XWnt8–Myc, and can displace 50% of Wg or 90% of XWnt8–Myc from a preformed complex with WIF-1–IgG in a 2-hour incubation at 4 °C (Fig. 4e).
Figure 4f shows that the binding efficiency of XWnt-8–Myc for hWIF-1 is comparable to that of a fusion protein between the CRD of Drosophila Frizzled-2 and IgG (Dfz2CRD–IgG). In the presence of a 40-fold excess of soluble mouse sFRP-3 (msFRP-3), there was less co-precipitation of XWnt8–Myc with Dfz2CRD–IgG but the co-precipitation of XWnt8–Myc with WIF-1–IgG was unaffected. Consistent with this, XWnt8–Myc co-precipitated with WIF-1–IgG several-fold more efficiently than it did with an IgG fusion proteincarrying the CRD of msFRP-3 (data not shown). By contrast, a 40-fold excess of soluble hWIF-1 significantly decreased the co-precipitation of XWnt8–Myc with Dfz2CRD–IgG and with WIF-1–IgG.
To quantify the binding affinity of hWIF-1 for XWnt8, a fusion of XWnt-8–Myc and human placental alkaline phosphatase (XWnt8–AP) was produced in S2 cells and used for binding to hWIF-1, WIF-1–IgG, or IgG coated onto 96-well plates. After 24 hours incubation at 4 °C, we detected little or no binding to IgG, and the hyperbolic binding curves for hWIF-1 and WIF-1–IgG were almost identical (Fig. 4g, left). A linear Scatchard plot of the binding data (Fig. 4g, right) is indicative of a simple non-cooperative interaction having a k D of 16 nM.
These results with soluble secreted proteins indicate that at least two Wnt proteins, Wg and XWnt8–Myc, can form a specific, high-affinity, non-covalent complex in vitro with hWIF-1, and that hWIF-1 and Dfz2CRD bind to sites on XWnt8–Myc that either overlap or show negative cooperativity. These experiments do not address the possibility that additional proteins derived from the S2-conditioned medium might be present in our Wnt/hWIF-1 or Wnt/CRD complexes.
These results indicate that Wnt proteins can interact with structurally different extracellular inhibitors, as has been shown for the bone morphogenetic proteins8,9,10. These extracellular inhibitors may fine-tune the spatial and temporal domains of Wnt effects or may act to transport Wnt proteins across the intercellular space, releasing them at the appropriate location11. The presence of EGF-repeat domains in WIF-1 and a heparin-binding domain in sFRPs (ref. 12 and A.R., J.-C.H. and J.N., unpublished results) suggest that the spatial distribution of these Wnt antagonists is tightly controlled. A further level of complexity can be envisaged if, as our results indicate, various antagonists differ in their relative affinities for different Wnt proteins. The biological importance of such a system of Wnt antagonists could be the creation of spatial and temporal patterns of Wnt activity that are more complex than those allowed by transcriptional control of Wnt gene expression alone.
Methods
Antibodies. Rabbit anti-WIF-domain antibodies were raised against a fusion protein composed of the T7 gene-10 protein fused to amino acids 29–168 of hWIF-1, and affinity-purified using an E. coli maltose-binding protein fused to the same WIF-1 segment. Rabbit anti-Wg antibodies were prepared by immunization with a fusion protein composed of amino acids 235–367 of Wg fused to glutathione-S -transferase (GST) and affinity-purified as described.
Protein production from transfected mammalian cells. The WIF-1–IgG fusion contains the entire hWIF-1 open reading frame, except for the C-terminal seven residues, upstream of the hinge region of the human IgG heavy-chain gene13. The WD–IgG fusion contains the first 180 residues of hWIF-1, a spacer of three glycines, and IgG. The Dfz2CRD–IgG fusion contains the first 271 residues of Drosophila Frizzled-2 upstream of IgG. hWIF-1 and the various IgG fusion proteins were produced in transiently transfected 293 cells. Serum-free conditioned medium was concentrated 20-fold by ultrafiltration. Control conditioned medium was prepared from untransfected 293 cells and similarly concentrated. Soluble msFRP-3 was purified from conditioned medium of transfected Chinese hamster ovary (CHO) cells that had been selected for methotrexate resistance following co-amplification of the msFRP-3 expression plasmid and a co-transfected dihydrofolate reductase plasmid. msFRP-3 in serum-free conditioned medium was purified to apparent homogeneity by heparin–Sepharose affinity chromatography.
Production of Wg, XWnt8–Myc and XWnt8–AP by S2 cells. Conditioned medium containing Wg was produced by heat-shocking S2 cells stably transfected with Wg cDNA under the control of a heat-shock promoter as described7. Conditioned medium containing XWnt8–Myc or XWnt8–AP was produced in stably transfected S2 cells by using a metallothionein promoter and incubating at 25 °C for 24 h in serum-free DES medium (Invitrogen) containing 0.5 mM CuSO4. The Wg- or XWnt8–Myc-containing conditioned medium was centrifuged at 100,000g at 4 °C for 1 h to remove aggregates. Control media were produced by heat shock or CuSO4 induction of untransfected S2 cells. Conditioned medium containing Wg and control conditioned medium for the Wg experiments were concentrated 20-fold by ultrafiltration. The XWnt8–Myc protein contains a C-terminal Myc-epitope tag (EQKLISEEDL) inserted between amino acids 339 and 340 (ref. 5); the XWnt8–AP construct contains alkaline phosphatase fused immediately beyond this Myc tag.
In situ hybridization. Whole-mount in situ hybridization was done as described14.
Xenopus embryo and oocyte microinjection. hWIF-1 was expressed from the pCS2+ expression vector. RNA synthesis and microinjection into Xenopus embryos have been described15.
Armadillo stabilization assays. Drosophila clone-8 cells, seeded one day earlier and grown to 80% confluence, were incubated with control or Wg-containing conditioned medium from S2 cells. Before incubation with clone-8 cells, the S2 conditioned medium (0.4 ml) was pre-incubated for 25 min at 4 °C with 0.4 ml DMEM/F-12 medium from transfected or control 293 cells. After 3 h at 25 °C, clone-8 cells were collected, washed in 1× PBS, 5 mM EDTA, and lysed in 80 µl hypotonic buffer (10 mM Tris, pH 7.5, 0.2 mM MgCl2) containing protease inhibitors. After addition of 20 µl 1.25 M sucrose, a membrane-free cytoplasmic fraction was prepared by centrifugation at 100,000g for 30 min at 4 °C, resolved by SDS–PAGE, immunoblotted and analysed for Armadillo (mAb N2-7A1; ref. 16), actin (Amersham) and HSP-70 (Sigma).
Solution binding assay for Wg and XWnt8–Myc. 200 µl 293-cell conditioned medium containing WIF-1–IgG, WD–IgG, or IgG (each adjusted by ultrafiltration to 60 nM) was incubated with protein A–Sepharose beads at 4 °C for 1 h, after which the beads were washed 3 times with PBS and then incubated with 400 µl Wg or XWnt8–Myc conditioned medium at 4 °C for 2 h. The beads were separated from unbound material by low-speed centrifugation and washed five times with PBS. Co-precipitates were analysed by SDS–PAGE and immunoblotted with affinity-purified rabbit anti-Wg antibodies or anti-Myc mAb 9E10 (ref. 17).
Quantitative binding of XWnt8–AP and hWIF-1. Conditioned medium (100 µl) containing WIF-1 (10 µg ml−1), WIF-1–IgG (5 µg ml−1), or IgG (4 µg ml−1) was used to coat 96-well plate at 4 °C overnight, followed by incubation at 4 °C for 4 h with 200 µl 2 mg ml−1 BSA in binding buffer (Hank's balanced salt, 20 mM HEPES, pH 7.0). 150 µl XWnt8–AP diluted in 2 mg ml−1 BSA in binding buffer was applied to each well and incubated at 4 °C for 24 h. After 5 washes with 200 µl each of binding buffer, bound XWnt8–AP was quantified by measuring alkaline phosphatase activity spectrophotometrically. A plot of alkaline phosphatase activity, representing the concentration of bound XWnt8–AP relative to the total concentration of XWnt8, was fitted to the simple binary binding model (A + B ↔ A·B).
References
Wodarz, A. & Nusse, R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 (1998).
Moon, R. T., Brown, J. D. & Torres, M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 13, 157–162 (1997).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Hoppler, S., Brown, J. D. & Moon, R. T. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 10, 2805–2817 (1996).
Christian, J. L. & Moon, R. T. Interactions between XWnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 7, 13–28 (1993).
Moon, R. T. et al. Dissecting Wnt signaling pathways and Wnt-sensitive developmental processes through transient misexpression analyses in embryos of Xenopus laevis. Development (suppl.), 85–94 (1993).
van Leeuwen, F., Harryman Samos, C. & Nusse, R. Biological activity of soluble Wingless protein in cultured Drosophila imaginal disc cells. Nature 368, 342–344 (1994).
Piccolo, S., Sasai, Y., Lu, B. & DeRobertis, E. M. Dorsoventral patterning in Xenopus : inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86, 589–598 (1996).
Zimmerman, L. B., De Jesus-Escobar, J. M. & Harland, R. M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86, 599–606 (1996).
Hsu, D. R., Economides, A. N., Wang, X., Eimon, P. M. & Harland, R. M. The Xenopus dorsalizing factor gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell 1, 673–683 (1998).
Cadigan, K. M., Fish, M. P., Rulifson, E. J. & Nusse, R. Wingless repression of Drosophila frizzled2 expression shapes the Wingless morphogen gradient. Cell 93, 767–777 (1998).
Finch, P. W. et al. Purification and molecular cloning of a secreted frizzled-related antagonist of Wnt signaling. Proc. Natl Acad. Sci. USA 94, 6770–6775 (1997).
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B. & Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313 (1990).
Rebagliati, M. R., Toyoma, R., Haffter, P. & Dawid, I. B. Cyclops encodes a nodal-related factor involved in midline signaling. Proc. Natl Acad. Sci. USA 95, 9932–9937 (1998).
He, X., Saint-Jeannet, J.-P., Woodgett, J. R., Varmus, H. E. & Dawid, I. B. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 374, 617–622 (1995).
Peifer, M., Orsulic, S., Sweeton, D. & Wieschaus, E. Arole for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development 118, 1191–1207 (1993).
Evan, G. I., Lewis, G. K., Ramsay, G. & Bishop, M. J. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5, 3610–3616 (1985).
Acknowledgements
We thank R. Moon for Xwnt8-Myc cDNA, J. Flanagan for the alkaline phosphatase plasmid, J. Corden for the mouse RNA polymerase II clone, B. Appel, L. Roman and D. Grunwald for cDNA libraries, and P. Bhanot and I. Munoz-Sanjuan for comments on the manuscript. Supported by the Howard Hughes Medical Institute (J.-C.H., A.R., P.M.S., C.H.S., R.N., J.N.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsieh, JC., Kodjabachian, L., Rebbert, M. et al. A new secreted protein that binds to Wnt proteins and inhibits their activites. Nature 398, 431–436 (1999). https://doi.org/10.1038/18899
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/18899
This article is cited by
-
Evaluating the effects of curcumin nano-chitosan on miR-221 and miR-222 expression and Wnt/β-catenin pathways in MCF-7, MDA-MB-231 and SKBR3 cell lines
Diagnostic Pathology (2024)
-
WNT-inhibitory factor 1-mediated glycolysis protects photoreceptor cells in diabetic retinopathy
Journal of Translational Medicine (2024)
-
Therapeutic progress and challenges for triple negative breast cancer: targeted therapy and immunotherapy
Molecular Biomedicine (2022)
-
Pathological oligodendrocyte precursor cells revealed in human schizophrenic brains and trigger schizophrenia-like behaviors and synaptic defects in genetic animal model
Molecular Psychiatry (2022)
-
Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.