Abstract
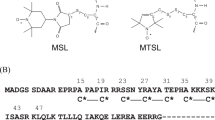
TROPOCOLLAGEN macromolecules, in appropriate reaction conditions, pack into the segment long spacing form (SLS) with the molecules arranged in parallel array and with like features in register1. SLS collagen thus provides an intensified and laterally extended picture of the tropocollagen molecule from which it is possible to determine by electron microscopy, after appropriate staining, axial distribution of those regions of the molecule rich in polar amino-acids. Comparison of the positions of these regions with the distribution observed in native (640 Å periodic) collagen fibrils allows the broad features of the fibril-assembly mechanism to be deduced2,3. To date the staining has been non-selective, presumably involving the binding of phosphotungstic acid (PTA) to the guanidino and amino side chains of arginine, lysine and hydroxylysine, or the uptake of chromium complexes and uranyl cations by the carboxylic groups of glutamic and aspartic acids4. Bensusan et al. claimed to have located the position of arginine residues using PTA staining of SLS prepared from solutions of collagen which had been 87 per cent acetylated to block the ε-amino group of lysine5. They argued that with so few histidines in the collagen molecule, the bonds observed after this treatment must reflect the position of the arginyl residues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schmitt, F. O., Gross, J., and Highburger, J. H., Symp. Soc. Exp. Biol., 9, 148 (1955).
Hodge, H. A., and Petruska, J. A., in Some Aspects of Protein Structure (edit. by Ramachandran, G. N. (Academic Press, New York, 1963).
Hodge, H. A., and Schmitt, F. O., Proc. US Nat. Acad. Sci., 46, 186 (1960).
Kuhn, K., and Zimmer, E., Z. für Naturforsch., 16B, 648 (1961).
Bensusan, H. B., Mumaw, U. R., and Scanu, A. W., Biochemistry, 1, 215 (1962).
Kuhn, K., Grassmann, W., and Hoffmann, U., Z. für Naturforsch., 13B, 154 (1958).
Sakaguchi, S., J. Biochem. (Tokyo), 5, 25 (1925).
Bhattacharya, K. R., Datta, J., and Roy, D. K., Arch. Biochem. Biophys., 77, 297 (1958).
Bowden, J., Chapman, J. A., and Wynn, C., Biochim. Biophys. Acta, 154, 190 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WEISS, J., BOWDEN, J. Spatial Recognition of Arginine in the Tropocollagen Macromolecule. Nature 222, 1266–1268 (1969). https://doi.org/10.1038/2221266a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/2221266a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.