Abstract
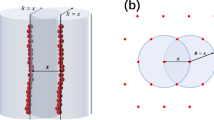
POLYELECTROLYTES are known to alter drastically the rate of many ionic reactions in solution1,2. In general, it may be said that the polyelectrolyte effect has an important role in the observed changes of the rates of reaction. The high electrostatic field in the neighbourhood of the macroion generates an inhomogeneous distribution of the ionic reactants—counterions being concentrated in a rather small volume surrounding the polymeric chains. It has been clearly shown3,4 that even when the ionic substrate is decomposed by specifically reactive groups incorporated into the polyelectrolyte molecule, the electrostatic interaction is the causative factor of the observed accelerated rate of decomposition. This polyelectrolyte effect may be a relevant factor on the activity of catalytic biopolyelectrolytes, for example enzymes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Morawetz, H., Accs Chem. Res., 3, 354–60 (1970).
Ise, N., Adv. Polymer Sci., 7, 536–93 (1971).
Fernández-Prini, R., and Turyn, D., J. Chem. Soc. Faraday I., 69, 1326–37 (1973).
Fernández-Prini, R., and Baumgartner, E., J. Am. Chem. Soc., 96, 4489–94 (1974).
Kabanov, V., Ptrovskaya, V., and Kargin, V., Vysokomolekul. Soedin, A 10, 925–31 (1968).
Overberger, C. G., and Podsiadly, C. J., Bioorg. Chem., 3, 16–34 (1974).
Applequist, J., and Doty, P., in Polyamino Acids, Polypeptides and Proteins, (edit. by Stahmann, M. A.), 161–77 (University of Wisconsin Press, Wisconsin, 1962).
Kirby, A. J., and Varvoglis, A. G., J. Am. Chem. Soc., 89, 415–23 (1967); J. Chem. Soc., (B), 135–41 (1968).
Kirby, A. J., and Jencks, W. P., J. Am. Chem. Soc., 87, 3209–16, 3217–24 (1965).
Townend, R., Kumosinski, T., Timasheff, S., Fasman, G., and Davidson, B., Biochem. biophys. Res. Commun., 23, 163–9 (1966).
Lehninger, A. L., Biochemistry, ch. 6 (Worth, New York, 1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BLESA, M., TURYN, D., FERNANDEZ-PRINI, R. et al. Influence of conformation on effect of macroions on kinetics of ionic reactions. Nature 256, 681–682 (1975). https://doi.org/10.1038/256681a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/256681a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.