Abstract
Study design:
Cross-sectional and longitudinal design.
Objectives:
(1) To quantify skeletal muscle cross-sectional area (CSA) after correcting for intramuscular fat (IMF) in thigh muscle groups 6 weeks after incomplete spinal cord injury (SCI), (2) to monitor the changes in muscle CSA and IMF after 3 months from the initial measurement.
Setting:
Academic institution Athens, GA, USA.
Methods:
Six incomplete SCI patients (28±4 years, 178±5 cm and 78±6 kg, mean±SE, C7 to L3, American Spinal Injury Association B or C) were tested at 5±1 weeks and 3 months after the initial measurement. T1-weighted magnetic resonance images were taken of both thighs. Six able-bodied (AB) controls were matched in age, sex, height and weight (29±4 years, four male and two female subjets, 179±5 cm and 77±6 kg).
Results:
At 6 weeks post-injury, muscle CSA was 82±4 cm2 in incomplete SCI and 123±21 cm2 in AB controls (P=0.04). IMF CSA was 5.2±1.3 and 2.3±0.6 cm2 in incomplete SCI and AB controls, respectively (P=0.03). Relative IMF was three-fold higher (P=0.03) in the SCI group versus AB controls (5.8±1.4 versus 2.0±0.6%). After 3 months, IMF increased 26% in the SCI group compared to the initial measurement (P=0.02).
Conclusions:
Skeletal muscle atrophy is associated with greater IMF accumulation in SCI group 6 weeks post-injury compared to AB controls. Moreover, IMF continues to increase over time in incomplete SCI.
Similar content being viewed by others
Introduction
Following spinal cord injury (SCI), the most prominent adaptation is skeletal muscle atrophy.1, 2, 3, 4 For example, skeletal muscle cross-sectional area (CSA) has been reported to be as much as 45% smaller than that of able-bodied (AB) controls just 6 weeks after a clinically complete SCI.2 Skeletal muscle atrophy has been attributed to the loss of central activation and subsequent unloading5 and is associated with adverse metabolic sequelae, including glucose intolerance, insulin resistance and later on type II diabetes.6, 7
Skeletal muscle atrophy has been shown to be related to the accumulation of intramuscular fat (IMF) in complete SCI, elderly and stroke patients.4, 8, 9 IMF can be defined as the sum of infiltrated fat within an individual muscle group (intra- and extramyocellular fat compartments) and intermuscular adipose tissue (IMAT). IMF should not be confused with IMAT, which is the visible fat infiltrated between muscle groups such as quadriceps femoris and hamstring muscles.10 It is not possible with computerized tomography and magnetic resonance imaging (MRI) to separate between intra- and extramyocellular fat compartments,8, 9, 11 but MRI could measure IMAT as it has been previously documented.10 It has recently been determined that in complete SCI IMF accounts for a 70% reduction in glucose tolerance.4 Goodpaster et al12 showed also that IMF is negatively correlated with insulin sensitivity in obese and non-obese subjects with and without type II diabetes.
Over half of the SCI population consists of individuals with incomplete SCI.13, 14 This group of patients often presents with impairments ranging from minimal sensory and motor loss below the level of lesion to complete motor loss with sacral sparing only.15 Skeletal muscle adaptations after acute incomplete SCI are not well documented. It has been reported that in order to accurately quantify the extent of muscle atrophy, skeletal muscle size needs to be corrected for IMF.4 Failure to correct for IMF could result in underestimation of muscle atrophy by ∼12% in SCI individuals.4
Owing to the fact that thigh muscle is relatively large and has been linked to metabolic disorders12, 16, 17 and skeletal muscle atrophy has been associated with increased IMF accumulation,4, 9 accurate quantification of muscle size and IMF a few weeks after incomplete SCI is of particular interest. In addition, early quantification of muscle atrophy reflects the importance of early interventions to defend muscle size. Early rehabilitation interventions should start when individuals with SCI become clinically stable.2 Additionally, while skeletal muscle atrophy may not be completely avoided, the length of the rehabilitation period should be enough to slow the progression of this process. Previous studies showed that 3 months of evoked resistance training were sufficient to increase muscle size.18 Therefore, the aims of this study were: (1) to measure skeletal muscle CSA few weeks after incomplete SCI after correcting for IMF, (2) to monitor the changes in skeletal muscle CSA and IMF content in incomplete SCI individuals 3 months after the initial measurement. We hypothesized that affected skeletal muscle CSA would be smaller and that IMF would be larger in SCI individuals compared with AB controls. Additionally, we hypothesized that skeletal muscle CSA would decrease while IMF would increase in incomplete SCI individuals during the 3 months after the initial measurement.
Methods
Subjects
Six acute, incomplete SCI and six AB controls participated in this study. The SCI individuals were classified according to American Spinal Injury Association (ASIA) as B or C with their level of injury ranging from C7 to L3. The physical and clinical characteristics of the SCI group are listed in Table 1. All SCI individuals were ‘incomplete’ with sacral sparing as confirmed by ASIA and they were wheelchair dependent with occasional ambulation. The SCI group was recruited from the Shepherd Center (Atlanta, GA, USA) approximately 6 weeks after injury when they were medically stable. Six (four male and two female subjects) AB controls were recruited and matched for gender, age, height and weight with the SCI group (Table 1). The AB controls were recruited from the population of the University of Georgia and the Shepherd Center. They were recreationally active but not engaged in a regular exercise program. Four were right-side dominant and the rest were left-side dominant as determined by self-report. T1-weighted magnetic resonance images of both thighs were taken of the SCI group at 5±1 weeks post-injury with follow up scans 3 months after the initial scans, which were performed at ∼4.5 months post-injury. In the control group, subjects were scanned once at the beginning of the study. The study was approved by the Institutional Review Boards of the University of Georgia and the Shepherd Center, and all participants provided written informed consent.
Magnetic resonance (MR) imaging
Skeletal muscle CSA was determined using proton-weighted MRI. This method is highly reproducible and reliable for determination of skeletal muscle composition.4, 8, 10 MR images of both thighs were acquired using a 1.5 Tesla magnet and a whole body coil. T1-weighted transaxial images, 1 cm thick and 0.5 cm apart, were taken from the hip to the knee joints (17 slices, TR/TE 500/14 ms, 40 cm field of view (FOV), one repetition and 256 × 256 matrix size). The location of the scan was specifically identified by placing a mark 6 inches proximal to the patella and matched for the follow up scan after 3 months for similar position in the magnet. The MR images were analyzed with X-Vessel software. The thigh images were automatically segmented into fat (high intensity), skeletal muscle (mid intensity) and background/bone (low intensity). A preliminary segmentation of each 2D slice was obtained by simplex optimization of the correlation between a Sobel-gradient image computed from the original image.19 This first-pass segmentation was used to correct for intensity variations across the original image caused by radio frequency heterogeneity.20 The corrected original image was then resegmented into the three intensity components using a fuzzy c-mean clustering algorithm.21 Manual selection of a pixel of skeletal muscle highlighted all skeletal muscle pixels and provided the total number of skeletal muscle pixels exclusive of fat or low-intensity pixels. Two trials on sample images were performed to determine the test–retest reliability (r=0.99).
Skeletal muscle and intramuscular fat calculations
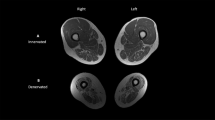
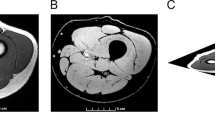
The procedure for calculating skeletal muscle CSA and IMF CSA has been previously described in detail.4 Briefly, the outer perimeter of the thigh muscle groups was traced and the femur bone was excluded (Figure 1). The region of interest was then highlighted and the signal intensity within this region was automatically determined (see above). After the pixel signal intensity was determined, a bimodal histogram of two peaks was plotted. The first peak represents the muscle peak and the second represents the fat peak. A mid-point between the two peaks separates muscle from fat pixels. The CSAs were calculated using the following equations: Muscle CSA (cm2)=total number of pixels of muscle*(FOV/matrix size)2 and IMF CSA (cm2)=total number of pixels of IMF*(FOV/matrix size)2. To control for the difference in skeletal muscle size between the groups, IMF CSA was expressed relative to skeletal muscle CSA. Relative IMF=((IMF CSA/muscle CSA)*100). In the control group, the nondominant limb was chosen to represent skeletal muscle and IMF CSA. In the SCI group, the involved thigh was chosen for statistical analysis. This was defined as the thigh with apparent muscle atrophy, MRI classified, after 6 weeks (Figure 2). The difference in average CSA between the involved and the less-involved thighs was not statistically different after both scans (see Results). However, this design was chosen because incompleteness of the injury could have influenced the degree of skeletal muscle atrophy to one side compared to the other. This assumption was based on the fact that incomplete SCI individuals tend to have a weaker side compared to the other side.22
Statistical analysis
Independent t-tests were used to test for any significant differences in physical characteristics, skeletal muscle CSA, IMF and relative IMF between the incomplete SCI patients at 6 weeks post-injury and the AB controls. Dependent t-tests were used to test for differences in IMF, skeletal muscle CSA and relative IMF, 3 months after the initial measurement for the incomplete SCI only. Data are presented as mean±standard error (SE).
Results
The physical characteristics of the SCI individuals and AB controls are presented in Table 1. The SCI group was 28±4 years, 178±5 cm and 78±6 kg, mean±SE. There were no differences in the age, height and body weight between incomplete SCI and AB control groups (P>0.05), indicating the effectiveness of matching between both groups. The duration of injury for SCI group was 5±1 weeks, range=3–7 weeks post-injury.
Skeletal muscle cross-sectional area
In the SCI group, skeletal muscle CSA of the involved side was not significantly different from the less-involved side, both at 6 weeks (82±4 and 84±5, P=0.3) and after 3 months (76±8 and 76±9, P=0.6). At 6 weeks after SCI and after correcting for IMF, the thigh skeletal muscle CSA was 33% smaller than AB controls (82±4 versus 123±21 cm2, P=0.04). Failure to correct for IMF resulted in an underestimation of skeletal muscle atrophy by ∼6% (82±4 versus 87±4 cm2, P=0.01). In the SCI group, thigh skeletal muscle CSA did not significantly change during the following 3 months (10%; 76±8 cm2, P=0.3; Figure 3).
Skeletal muscle CSA in AB controls and incomplete SCI individuals 6 weeks after injury (black and gray columns) with follow up scan after 3 months (gray column, SCI only at 4.5 months post-injury). *Indicates significant difference from AB controls at 6 weeks and 3 months. No significant difference in SCI at 6 weeks and 3 months scans
Intramuscular fat cross-sectional area
In the SCI group, the IMF CSA of the thigh was 126% larger 6 weeks post-injury compared to that of the AB controls (5.2±1.3 versus 2.3±0.6 cm2, P=0.03). Following 3 months, IMF CSA increased 26% in SCI individuals (5.2±1.3 to 7.2±1.5 cm2, P=0.02; Figure 4).
Relative intramuscular fat
Relative IMF (calculated as (IMF CSA/muscle CSA)*100) was three-fold higher in the SCI group compared to the AB group at 6 weeks (5.8±1.4 versus 2.0±0.6%, P=0.03). In the subsequent 3 months, relative IMF continued to increase in the SCI individuals (8.6±2.5%; P=0.02).
Discussion
This is the first study to investigate skeletal muscle adaptations during the acute phase of incomplete SCI after correcting for IMF. The primary findings of this study were that thigh skeletal muscle CSA was 33% lower in incomplete SCI individuals compared to their matched AB controls, whereas IMF content was 126% greater in SCI patients 6 weeks post-injury. Failure to correct for IMF results in underestimation of muscle atrophy. Furthermore, IMF content increased by an additional 26% during the 3 months follow up in SCI patients.
These data are in agreement with previous findings from our laboratory. Castro et al2 showed a marked atrophy in skeletal muscle CSA a few weeks post-injury in SCI compared to AB controls. Elder et al4 has recently documented a marked increase in IMF in chronic complete SCI individuals compared to AB controls, an increase that was strongly correlated with glucose intolerance in this population. Previous research showed that unloading results in skeletal muscle atrophy.23, 24 For example, knee extensor muscle CSA was reduced by 14% following 6 weeks of bed rest,23 and the thigh muscle CSA decreased 12% after 6 weeks of unilateral lower limb suspension.24 The magnitude of skeletal muscle atrophy reported in this study indicates that incomplete SCI could evoke greater disuse than previous reported models of microgravity. Furthermore, skeletal muscle atrophy was even more pronounced by 6% after correcting for IMF. There was also a reduction in muscle size by 10% after 3 months, which would probably be of clinical significance although it did not attain statistical significance. In healthy individuals, a 5% loss in muscle mass can impair organ function and a loss of 20% or more can cause organ failure and death.25 This demonstrates the importance of correcting for IMF when referring to muscle size in clinical populations. Surprisingly, the magnitude of muscle atrophy was not different between the visually determined involved and less-involved thighs. These findings suggest that neural drive is responsible for the one-side weakness that commonly occurs with incomplete SCI and differing muscle atrophy is not a major contributor.
It should be noted that the time course of evaluation (6 weeks post-injury and 3 months after the initial scan) is of clinical importance in context of interventions to defend muscle size. Individuals with incomplete SCI could not be involved in any intervention until they were clinically stable; this turned out to be on average 6 weeks post-injury. Castro et al2 showed that the greatest magnitude of skeletal muscle atrophy occurs 3 months after the 11th week scans. In addition, previous studies showed that effective resistance training to increase muscle strength and size would last approximately 3 months.18, 26 In complete SCI, 3 months of evoked resistance training result in skeletal muscle hypertrophy up to 40%.18 Absence of effective intervention within this period may result in the clinical consequences demonstrated in this study. Therefore, clinicians administering interventions should be aware of the time course required to attain maximum rehabilitation goals.
Body composition changes after SCI may be a contributing factor for the increased risk of developing obesity, type II diabetes and metabolic syndrome.6, 7 Skeletal muscle atrophy after injury is theoretically linked to reductions in total energy expenditure and energy imbalance resulting in obesity and glucose intolerance; predisposing individual with SCI to an increased risk of cardiovascular diseases and type II diabetes.7 Data to support this link is available in chronic SCI who increased muscle CSA by 40% results in enhancing glucose disposal after 12 weeks of electrically stimulated resistance training.18
The higher prevalence of insulin resistance in spinal cord-injured individuals compared to controls is well established.6, 27 Several factors such as body composition, age since injury and physical inactivity have been shown to contribute to the higher rate of insulin resistance after SCI.27 IMF is a better predictor of insulin resistance in nondisabled, obese diabetics and nondiabetics compared to subcutaneous and subfascial fat.12 Moreover, IMF explains 70% of glucose intolerance after chronic SCI.4 The mechanism relating IMF and insulin resistance is not clear; however, the release of intracellular fatty acid intermediates (eg, diacylglycerol, ceramide, long-chain acyl-CoA) from IMF could impair insulin signaling.28 In the SCI group, the increase in IMF was ∼7 cm2 3 months after the initial scan, which represents 50% of the reported value (14 cm2) in chronic SCI over an 8-year period.4 The mechanism behind IMF accumulation is not clear, but failure of skeletal muscle to utilize glucose after paralysis may inhibit the process of fatty acid oxidation and result in greater IMF accumulation.29, 30 Accumulation of IMF and its possible role in explaining insulin resistance demonstrate the importance of early interventions after SCI as well as the search of modalities that can attenuate muscle atrophy and preventing fat accumulation.
In the present study, MRI was used to separate between muscle and fat pixels based on their signal intensities. Previously, MRI was used to quantify skeletal muscle response to unloading and after SCI.1, 2, 3, 4, 23, 24 The accuracy of MRI versus dual-energy X-ray absorptiometery (DEXA) in determining skeletal muscle atrophy has been previously investigated showing that DEXA overestimates the proportion of the muscle in fat-free mass after SCI and inaccurately reflects the degree of muscle atrophy compared to MRI.3 MRI also provides the advantage over computed tomography (CT) by facilitating the acquisition of multiple slices over a single slice commonly used in CT scans, which can inaccurately represent the exact changes in muscle size in response to training or disuse. Furthermore, MRI protects against the risk of ionizing radiation with exposure to CT.
One of the potential limitations to the current study is matching SCI and AB controls, which is difficult due to differing height, weigh and muscle CSA relations in SCI. However, every effort has been made to match both groups for age, height, weight and sex as has been done in similar studies.2, 3, 4 The small sample size (n=6), the variability in level of injury (C7–L3) and ASIA classification (B and C) could also be considered limitations of the current study. This study was conducted to provide supportive pilot data for future studies with a reasonable sample size that could investigate possible relation between IMF and insulin resistance in incomplete SCI. It should be noted that the effect size according to the current data was greater than two standard deviations; this may have helped to attain a statistical difference with the current sample size. Additionally, the high precision of the MRI protocol used in the study undoubtedly increased statistical power; especially the error associated with this assessment tool is less than 1%. Furthermore, irrespective of level of injury and ASIA classification, the magnitude of skeletal muscle atrophy was not significantly or clinically different between an ASIA B (77 cm2) or C (76.5 cm2) individuals after 3 months from the initial measurement. This was further supported in the current study by the less than 10% standard error of the mean in SCI skeletal muscle size. Further studies, with a larger sample size, may be warranted to address the effect of level of injury and ASIA classification on muscle size after complete and incomplete SCI.
In summary, this study showed that individuals with incomplete SCI experience skeletal muscle atrophy as early as a few weeks post-injury with further decline after ∼4.5 months post-injury. Also failure to correct for IMF results in underestimation of skeletal muscle atrophy. The process of skeletal muscle atrophy is associated with higher IMF in the incomplete SCI group compared to their matched AB controls. Furthermore, IMF accumulation continued to increase over a 3-month period. Because muscle atrophy and high IMF content are associated with increased risk for metabolic disease, attempts to reverse these processes in SCI patients may have very important health benefits. Therefore, our findings suggest that early intervention (ie, beginning at least a few weeks after injury) in individuals with incomplete SCI may be important to reduce skeletal muscle atrophy and IMF accumulation. Further studies should address the relationship between skeletal muscle atrophy, IMF accumulation and increased risk of developing type II diabetes in this population.
References
Dudley GA, Castro MJ, Rogers S, Apple Jr DF . A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol 1999; 80: 394–396.
Castro MJ, Apple Jr DF, Hillegass EA, Dudley GA . Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol 1999; 80: 373–378.
Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA . Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol 2004; 96: 561–565.
Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA . Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord 2004; 42: 711–716.
Gordon T, Mao J . Muscle atrophy and procedures of training after spinal cord injury. Phys Ther 1994; 74: 50–60.
Bauman WA, Spungen AM . Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001; 24: 266–277.
Kocina P . Body composition of spinal cord injured adults. Sports Med 1997; 23: 48–60.
Kent-Braun JA, Ng AV, Young K . Skeletal muscle contractile and non-contractle composition in younger and older women and men. J Appl Physiol 2000; 88: 662–668.
Ryan AS, Dobrovolony CL, Smith GV, Silver KH, Macko RF . Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil 2002; 83: 1703–1707.
Gallagher D et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005; 81: 903–910.
Ross R, Goodpaster BH, Kelley DE, Boada F . Magnetic resonance imaging in human body composition research: from quantitative to qualitative tissue measurement. Ann NY Acad Sci 2000; 904: 12–17.
Goodpaster BH, Thaete FL, Kelley DE . Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000; 71: 885–892.
Lapointe R, Lajoie Y, Serrese O, Barbeau H . Functional community ambulation requirements in incomplete spinal cord injured subjects. Spinal Cord 2001; 39: 327–335.
Sekon L, Fehlings M . Epidemiology, demographics and pathophysiology of acute spinal cord injury. Spine 2001; 26: S2–S12.
Maynard Jr FM et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
Ryan AS, Nicklas BJ . Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular diseased risk factors. Intl J Obes Relat Metab Disord 1999; 23: 126–132.
Heymsfield SB et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 1990; 52: 214–218.
Mahoney ET et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 2005; 86: 1502–1504.
Gonzalex RC, Wintz P . Digital Image Processing. Addison-Wesely: Reading, MA 1987.
Cohen MS, Dubois RM, Zeineh NM . Rapid and effective correction of RF inhomogenity for high field magnetic resonance imaging. Hum Brain Mapp 1987; 10: 204–211.
Suckling J, Siggmundsson T, Greenwood K, Bullmore ET . A modified fuzzy clustering algorithm for operator independent brain tissue classification of dual echo MR Images. Magn Reson Imaging 1999; 17: 1065–1076.
Jayaraman A et al. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord [E-pub ahead of print: 13 December 2005; doi:10.1038/sj.sc.3101892].
Berg HE, Larsson L, Tesch PA . Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol 1997; 82: 182–188.
Hather BM, Adams GR, Tesch PA, Dudley GA . Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol 1992; 7: 1493–1498.
Demling RH, DeSanti L . Involuntary weight loss and the nonhealing wound: the role of anabolic agents. Ad Wound Care 1999; 12: 1–14.
Ploutz LL, Tesch PA, Biro RL, Dudley GA . Effect of resistance training on muscle use during exercise. J Appl Physiol 1994; 76: 1675–1681.
Bauman WA, Spungen AM . Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994; 43: 749–756.
Yu C et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002; 277: 50230–50236.
Aksnes AK, Hjeltnes N, Wahlstrom EO, Katz A, Zierath JR, Wallberg-Henriksson H . Intact glucose transport in morphologically altered denervated skeletal muscle from quadriplegic patients. Am J Physiol 1996; 271: E593–E600.
Sidossis LS, Stuart CA, Shulman GL, Lopaschuk GD, Robert RW . Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry in the mitochondria. J Clin Invest 1996; 98: 2244–2250.
Acknowledgements
We thank all the participants. This study was supported by NIH grants HD37439-S (GAD) and HD39676 (GAD). We thank Dr Ronald Meyer for providing the software that was used to analyze the images for this study. Also, we acknowledge Drs Chris Feak and Jeff Horowitiz for their valuable comments during the preparation of the manuscript. Ashraf S Gorgey is currently supported by the National Institute on Disability and Rehabilitation Research (NIDRR) Grant #H133P03004.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gorgey, A., Dudley, G. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 45, 304–309 (2007). https://doi.org/10.1038/sj.sc.3101968
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101968
Keywords
This article is cited by
-
A comprehensive look at the psychoneuroimmunoendocrinology of spinal cord injury and its progression: mechanisms and clinical opportunities
Military Medical Research (2023)
-
Fat infiltration in the thigh muscles is associated with symptomatic spinal stenosis and reduced physical functioning in adults with achondroplasia
Orphanet Journal of Rare Diseases (2023)
-
Intensity of overground robotic exoskeleton training in two persons with motor-complete tetraplegia: a case series
Spinal Cord Series and Cases (2023)
-
Genetic diversity modulates the physical and transcriptomic response of skeletal muscle to simulated microgravity in male mice
npj Microgravity (2023)
-
Denervation impacts muscle quality and knee bone mineral density after spinal cord injury
Spinal Cord (2023)