Abstract
Study design:
Literature review.
Objective:
To describe quantitatively some of most important anatomic, systemic, and metabolic changes occurring soon (one month) after spinal cord trauma in mice.
Setting:
University Laval Medical Center.
Results:
Significant changes in weight, mechanical and contractile muscle properties, bone histomorphometry and biomechanics, deep-vein morphology, complete blood count, immune cell count, lipid metabolism and anabolic hormone levels were found occurring within 1 month in completely spinal cord transected (Th9/10) mice.
Conclusion:
These data reveal that many changes in mice and humans are comparable suggesting, in turn, that this model may be a valuable tool for neuroscientists to investigate the specific mechanisms associated with rapid health degradation post-SCI.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) leads generally to an irreversible loss of motor control and sensations below the level of trauma. In recent years, the secondary consequences and complications associated with chronic SCI have received increasing attention. Indeed, it is now well recognized that SCI patients, in particular those with complete or near-complete lesions, develop important and often life-threatening complications after their accident. For instance, muscle wasting, osteopenia or osteoporosis, hormone dysregulation, cardiovascular problems and immune deficiency are among the problems typically encountered by chronic SCI individuals.1, 2, 3, 4 Although many of these complications occur soon after trauma, little is known about the detailed mechanisms underlying their development and progression. Furthermore, no animal model had been characterized to specifically study these health problems. Since mice are increasingly recognized as offering clear molecular and genetic advantages over other species, we chose paraplegic mice as an animal model to study the many anatomic and metabolic changes associated with health degradation after SCI. In this article, we summarize recently published data mainly from our laboratory reporting changes in weight, mechanical and contractile muscle properties, bone histomorphometry and biomechanics, deep vein morphology, complete blood count, immune cell count, lipid metabolism and anabolic hormone levels.
Animal model
All data reported in this review, that are from our laboratory, were obtained using a completely spinal cord transected (Tx) mouse model.5, 6, 7, 8 All experimental procedures were conducted in accordance with the Canadian Council for Animal Care guidelines and accepted by the Laval University Animal Care, Use and Ethics Committee. In brief, adult male CD1 mice (Charles River Canada, St Constant, Quebec) weighing 30–40 g were used. Preoperative cares included subcutaneous injection of lactate-Ringer's solution (1 ml), analgesic (0.1 mg kg−1, Buprenorphine) and antibiotic (5 mg kg−1, Baytril). All surgical procedures were performed under aseptic conditions in deeply anesthetized animals (2.5% isoflurane). After exposing the area between the 6th and the 12th thoracic level, the spinal cord was completely transected using microscissors inserted between the 9th and 10th thoracic vertebrae. The opened skin area was then sutured and animals were placed for a few hours on heating pads. Post-operative cares included subcutaneous injection of lactate-Ringers's solution (2 × 1 ml day−1), buprenorphine (0.2 mg kg−1 day−1) and Baytril (5 mg kg−1 day−1). Complete spinal cord Tx was confirmed by (1) full paralysis of the hindlimbs initially, (2) post-mortem visual and microscopic examination of the spinal cord lesions and (3) histological examination of coronal or mid-sagittal spinal cord sections stained with luxol fast blue/cresyl violet for myelinated axons and Nissl substance, respectively. Only data from complete spinal cord Tx animals were used for analyses.
Results and discussion
Body weight
After monitoring weekly body weights for 1 month, we found a rapid and significant reduction in body weight in complete Tx mice (N=29, one animal died after surgery). A sudden loss that reached 24% (P<0.001) after only 7 days was monitored (see Table 1).5 Body weight values did not return to normal levels after 4 weeks. Post-mortem measurements of individual limb mass and volume revealed greater losses beneath lesion level compared with above lesion level. Indeed, at 7 days post-Tx, hindlimb mass and volume decreased by 28% whereas, a 21% reduction was found in the forelimbs (N=8, P<0.001 and P<0.05, respectively). A partial return to near normal values was found in the forelimbs but not in the hindlimbs at 4 weeks post-Tx. These relatively rapid adaptive changes are somewhat comparable to what has been observed generally in patients. A significant weight loss has indeed been reported in patients soon after their accident.9 Reasons underlying this initial weight loss are not fully understood. It is unclear, for instance, the extent to which reduced physical activity, metabolic changes or hormone dysregulations may play a role in this phenomenon. The decrease in hindlimb size may be partially explained by muscular atrophy due to reduced muscle activity and paralysis per se (see section on Hindlimb muscles). However, the forelimb atrophy, which was somehow unsuspected in paraplegic animals, suggests that factors other than reduced muscular activity (that is, forelimbs typically remained active to move around and to reach for food and water) contribute also to induce these rapid changes (see also section on Hormone dysregulation). However, after a few years, this early weight loss is often transformed into a weight gain. In fact, SCI patients generally tend to progressively undergo an increase in weight (mainly fat tissue increase) leading to overweight and obesity problems.9
Hindlimb muscles
As mentioned above, the hindlimbs are specifically affected in complete paraplegic mice. Results from our laboratory have demonstrated that part of this weight loss is due to a specific decrease in muscle mass. Soleus muscles were dissected out (N=8), weighed and tested in vitro for muscle properties. At 1 week post-Tx, a 32% (P<0.001) loss in mass was already detected in soleus muscles (Table 1).5 Similar values were found several weeks post-Tx. This decrease in mass corresponded also to a proportional decrease in strength. Indeed, using a set-up specifically designed for electrical muscle stimulation (using an electromagnetic field) and force-generating measurements in vitro, we found at 1 week post-Tx, a 33% (P<0.001) decrease (43% at 2 week post-Tx) in absolute maximal tetanic force (Po), combined with a 21% (26% at 2 week post-Tx) and 48% (P<0.05) increase in time-to-peak tension and half-time relaxation (½ RT), respectively.5 Studies in rats have reported that fiber-type conversion (slow oxidative to fast oxidative) may be induced later on after Tx.10, 11 Slow-twitch fibers (type I) in the Soleus were found to progressively acquire some of the biochemical profile and contractile properties of fast-twitch fibers (type IIa or IIb) after 3 months post-SCI. In turn, Po values in late chronic Tx rats were similar to those seen in early Tx mice. Since our results showed increased time to peak tension and ½ RT values at 1 and 2 weeks, but a return towards control values at 4 week post-Tx, data from late chronic rats and early chronic mice together provide evidence of bi-phasic changes in muscle property after Tx (that is, early transient increase in time to peak tension and ½ RT values followed by a sustained decrease). While the early transient changes in contraction and relaxation times are likely due to rapid alterations in Ca2+-induced-Ca2+-release mechanism, ryanodine and dihydropyridine receptors expression and free cytosolic Ca2+ concentration (see section Discussion),5 longer-term changes are most probably caused by slower mechanisms including fibre-type conversion and protein degradation (that is, triggered by increased calpain, lysosomal and ubiquitin-mediated proteolysis). In chronic SCI patients, biopsies and physiological tests using leg muscles have revealed comparable changes (that is decreased muscle strength and size, conversion to fast-twitch properties, and so on) to those in chronic Tx animals.12, 13, 14, 15 However, additional data from early SCI patients (less than 1 month) would be required before concluding that bi-phasic contractile property changes (that is time to peak tension and ½ RT), as seen in mice, exist also in humans.
Femoral bones
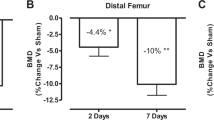
It is well-documented that SCI is associated with increasing risks of fracture. In fact, nearly all SCI individuals experience a significant loss of bone mineral tissue (up to 30% in the femora) leading to a marked increase of fracture incidence.16, 17 In Tx mice (N=12), histomorphometric data from our laboratory revealed a drastic decrease in trabecular bone volume (−22%, P=0.02), thickness (−11%, P=0.04) and number (−15%, P=0.09) within 10–30 days post-trauma (Table 1).6 Densitometric measurements using dual-energy X-ray absorptiometry on the femoral bones of Tx mice (N=14) reported no change in bone mineral density but a 14% reduction (P<0.001) in bone mineral content. Other models of disuse and immobilization have also reported comparable bone losses. For instance, a 10–30% decrease in femoral cancellous tissue was found within a few weeks (up to 50% after 18 weeks) of unilateral hindlimb immobilization in adult female rats.18 It remains unclear to what extent, rapid bone loss in SCI patients (most of which are young adults) share similar mechanisms with hormone-related osteopenia or osteoporosis seen in elderly people. However, given that clear differences in bone loss progression has been observed between animal models of disuse and age/hormone-related models, this suggests that differences in bone remodelling mechanisms may exist between young immobilized patients and elderly people. In fact, a murine model of disuse (hindlimb immobilization with a cast) provided evidence suggesting that bone loss occurring within a few days to a few weeks post-immobilization involves both a sharp decrease of osteoblastic activity and a rapid increase of osteoclastic activity (that is, low osteocalcin and high acid phosphatase levels).19 Further studies in mice (for example, SCI, cast-immobilization, tail-suspension) are likely to provide new insights into the molecular mechanisms of rapid bone loss after paralysis or immobilization.
Deep-vein size and blood lipid profile
Spinal cord injury is also associated with the development of deep-venous thrombosis (DVT) in the lower limbs and, hence, with rapidly increasing risks of cardiovascular and pulmonary complications soon after trauma.20 However, the specific mechanisms underlying DVT formation following SCI remain poorly understood. Using in vivo confocal microscopy, we recently established that deep vein changes in size can be found as soon as at 1 week post-Tx in mice.7 In fact, the femoral and saphenous veins were found to undergo a large increase (>1.5-fold) in diameter (P<0.01). This change in venous diameter remained similar for the entire period studied (4 weeks) (N=20). In this same study, we also analyzed the blood lipid profile using a clinical chemistry analyzer (Olympus AU400, Melville, NY, USA). We found also during the same period decreased concentrations of cholesterols (−25%), triglycerides (up to −45%), low-density lipoproteins (LDL, up to −55%), high-density lipoproteins (HDL, up to −14%) but not platelets (N=40). These results may appear surprising since high LDL-triglyceride levels are generally associated with DVT formation in humans. However, comparable data were found in patients, since acute chronic SCI subjects were found also to display low LDL-triglyceride levels.21 Results in acute SCI patients and early Tx mice suggest that LDL-triglyceride changes are unlikely to contribute to DVT formation soon after trauma. If changes in deep-vein size, as seen in Tx mice, were to be found also in patients, it would strongly suggest that deep-vein enlargement is a leading factor in DVT formation after SCI. We know, indeed, that venous stasis (reduced blood flow) is a key factor in DVT formation after immobilization20 and, interestingly, it has been associated with blood vessel enlargement in pregnant women.22
Blood and bone marrow cell counts
Immune deficiency may lead to life-threatening complications after SCI. To examine possible factors that may be associated with this pathological condition, we characterized using a CELL-DYN 3700 automatic blood analyzer (Abbott Laboratories, North Chicago, IL, USA) changes in red and white blood cells after Tx in adult CD1 mice (N=40). A complete blood count revealed unchanged or moderately decreased erythrocyte, platelet, hemoglobin and hematocrit levels.8 In contrast, leukocyte counts were greatly reduced in Tx mice compared with controls. Total leukocyte numbers decreased by 35% (P=0.002) at 1 week post-Tx and remained low at 2, 3 and 4 weeks (P<0.05). A detailed analysis of leukocyte subtypes including lymphocytes, monocytes, neutrophils and eosinophils, revealed the existence of differential modulatory changes. Lymphocyte numbers were reduced by 47% (P<0.001) on average (as much as 53% at 1 week post-Tx). Monocytes and neutrophils generally remained unchanged whereas eosinophil counts gradually decreased by 81% (P=0.027) after 4 weeks. Analyses from bone marrow samples revealed comparable changes. We found a general decrease in lymphocytes and mixed changes in neutrophils, monocytes and megakaryocytes after Tx.8 These results can be compared, to some extent, with those from SCI patients where reduced lymphocyte levels (specifically lymphocytes-T and NK cells) were found at 3 months post-SCI,23 which may perhaps contribute to the state of immune deficiency found generally in SCI patients.4, 24
Hormone dysregulation
Serum levels of testosterone, GH, DHEA, PTH and insulin were examined using ELISA in control and Tx mice at 7, 14, 21 or 28 days post-Tx (N=40). We found early transient changes in testosterone (decreased) and GH (increased) levels during the first 2 weeks post-Tx.8 In contrast, DHEA, PTH and insulin levels were reduced throughout the time period studied. Specifically, levels of testosterone in Tx mice were reduced by 40 and 50% at 1 and 2 weeks post-Tx, respectively. However, at 3 and 4 weeks post-Tx, testosterone concentration returned to near normal values with average serum levels ranging from 12.08 to 12.83 ng ml−1. In contrast, GH serum levels drastically increased at 1 week post-Tx with an average level three times greater than control animals (357.5 ng ml−1). However, at 2, 3 and 4 weeks post-Tx, GH concentration returned to near normal levels with values ranging from 363.9 to 417.6 ng ml−1. On the other hand, while testosterone and GH levels were only transiently changed, those of PTH, DHEA and insulin were diminished for the entire time period studied. Specifically, insulin levels were reduced by 84.5% at 1 week post-Tx and remained low. DHEA serum levels were reduced by as much as 75% a few weeks after Tx. Similar reductions were found with PTH levels. Comparable changes have been found in patients. Acutely injured men were found to display decreased serum testosterone during the first few weeks post-injury.25 Increased GH and decreased PTH levels have also been reported in chronic SCI patients.26 It remains unclear what role these hormonal changes may play in health degradation post-SCI. However, results in mice suggest that some of these changes may participate to immune deficiency since high correlations were found between specific anabolic hormone and immune cell type changes. For instance, the transient increase of GH was strongly correlated with changes in blood monocyte and megakaryocyte levels. This is also supported by data from the literature showing that GH receptors are expressed in peripheral mononuclear cell types and that GH can increase macrophageal activity and stimulate progenitor cell hematopoiesis.27, 28, 29 Regarding insulin and PTH, their sustained decrease post-Tx was highly correlated with the decrease in total blood leukocytes and lymphocytes. This finding is supported by results showing that (1) their receptors are expressed in leukocytes including lymphocytes and, (2) these hormones can stimulate lymphocyte synthesis in vivo.30, 31, 32, 33
Conclusion
This review article has reported essentially recent data from a mouse model of SCI. Anatomic, systemic and metabolic changes were found to rapidly occur in adult mice after a transection of the spinal cord at the low-thoracic level (complete paraplegia). To some extent, the early changes found in paraplegic mice were comparable to those reported in SCI patients. This supports the idea that a detailed characterization of health degradation in this animal model may provide the basis for additional studies and, hence, contribute to understand further the molecular and cellular changes underlying health degradation in patients. This work may eventually contribute to the development of new therapeutic approaches aimed at preventing these changes and life-threatening complications after SCI.
References
Giangregorio L, McCartney N . Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med 2006; 29: 489–500.
Bauman WA, Kahn NN, Grimm DR, Spungen AM . Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord 1999; 37: 601–616.
Bauman WA, Spungen AM . Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am 2000; 11: 109–140.
Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS . Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med 2000; 23: 129–135.
Landry E, Frenette J, Guertin PA . Body weight, limb size, and muscular properties of early paraplegic mice. J Neurotrauma 2004; 21: 1008–1016.
Picard S, Lapointe NP, Brown JP, Guertin PA . Histomorphometric and densitometric changes in the femora of spinal cord transected mice. J Spinal Cord Med (in press).
Rouleau P, Guertin PA . Early changes in deep vein diameter and biochemical markers associated with thrombi formation after spinal cord injury in mice. J Neurotrauma 2007; 24: 1406–1414.
Rouleau P, Ung RV, Lapointe NP, Guertin PA . Hormonal and immunological changes in mice after spinal cord injury. J Neurotrauma 2007; 24: 367–378.
Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G . Energy expenditure after spinal cord injury: An evaluation of stable rehabilitating patients. J Trauma 1985; 25: 419–423.
Lieber RL, Johansson CB, Vahlsing HL, Hargens AR, Feringa ER . Long-term effects of spinal cord transection on fast and slow rat skeletal muscle. I. Contractile properties. Exp Neurol 1986; 91: 423–434.
Talmadge RJ, Roy RR, Caiozzo VJ, Edgerton VR . Mechanical properties of rat soleus after long-term spinal cord transection. J Appl Physiol 2002; 93: 1487–1497.
Grimby G, Broberg C, Krotkiewska I, Krotkiewski M . Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med 1976; 8: 37–42.
Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R . Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord 1997; 35: 86–91.
Gerrits HL, De Haan A, Hopman MT, van Der Woude LH, Jones DA, Sargeant AJ . Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle Nerve 1999; 22: 1249–1256.
Scott WB, Lee SC, Johnston TE, Binkley J, Binder-Macleod SA . Contractile properties and the force–frequency relationship of the paralyzed human quadriceps femoris muscle. Phys Ther 2006; 86: 788–799.
Ragnarsson KT, Sell GH . Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 1981; 62: 418–423.
Zehnder Y, Lüthi M, Michel D, Knecht H, Perrelet R, Neto I et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004; 15: 180–189.
Li XJ, Jee WS, Chow SY, Woodbury DM . Adaptation of cancellous bone to aging and immobilization in the rat: a single photon absorptiometry and histomorphometry study. Anat Rec 1990; 227: 12–24.
Rantakokko J, Uusitalo H, Jamsa T, Tuukkanen J, Aro HT, Vuorio E . Expression profiles of mRNAs for osteoblast and osteoclast proteins as indicators of bone loss in mouse immobilization osteopenia model. J Bone Miner Res 1999; 14: 1934–1942.
Waring WP, Karunas RS . Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia 1991; 29: 8–16.
Apstein MD, George BC . Serum lipids during the first year following acute spinal cord injury. Metabolism 1998; 47: 367–370.
Macklon NS, Greer IA, Bowman AW . An ultrasound study of gestational and postural changes in the deep venous system of the leg in pregnancy. Br J Obstet Gynaecol 1997; 104: 191–197.
Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E . Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res 1992; 11: 104–116.
Nash MS . Known and plausible modulators of depressed immune functions following spinal cord injuries. J Spinal Cord Med 2000; 23: 111–120.
Naftchi NE, Viau AT, Sell GH, Lowman EW . Pituitary–testicular axis dysfunction in spinal cord injury. Arch Phys Med Rehabil 1980; 61: 402–405.
Mechanick JI, Pomerantz F, Flanagan S, Stein A, Gordon WA, Ragnarsson KT . Parathyroid hormone suppression in spinal cord injury patients is associated with the degree of neurologic impairment and not the level of injury. Arch Phys Med Rehabil 1997; 78: 692–696.
Kiess W, Butenandt O . Specific growth hormone receptors on human peripheral mononuclear cells: re-expression, identification, and characterization. J Clin Endocrinol Metab 1985; 60: 740–746.
Edwards III CK, Arkins S, Yunger LM, Blum A, Dantzer R, Kelley KW . The macrophage-activating properties of growth hormone. Cell Mol Neurobiol 1992; 12: 499–510.
Blazar BR, Brennan CA, Broxmeyer HE, Shultz LD, Vallera DA . Transgenic mice expressing either bovine growth hormone (BGH) or human gh releasing hormone (HGRH) have increased splenic progenitor cell colony formation and DNA synthesis in vitro and in vivo. Exp Hematol 1995; 23: 1397–1406.
Helderman JH, Strom TB . Specific insulin binding site on T and B lymphocytes as a marker of cell activation. Nature 1978; 274: 62–63.
Atkinson MJ, Hesch RD, Cade C, Wadwah M, Perris AD . Parathyroid hormone stimulation of mitosis in rat thymic lymphocytes is independent of cyclic amp. J Bone Miner Res 1987; 2: 303–309.
Walrand S, Guillet C, Gachon P, Giraudet C, Rousset P, Vasson MP et al. Insulin regulates protein synthesis rate in leukocytes from young and elderly healthy humans. Clin Nutr 2005; 24: 1089–1098.
Perry III HM, Chappel JC, Bellorin-Font E, Tamao J, Martin KJ, Teitelbaum SL . Parathyroid hormone receptors in circulating human mononuclear leukocytes. J Biol Chem 1984; 259: 5531–5535.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ung, RV., Lapointe, N. & Guertin, P. Early adaptive changes in chronic paraplegic mice: a model to study rapid health degradation after spinal cord injury. Spinal Cord 46, 176–180 (2008). https://doi.org/10.1038/sj.sc.3102119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102119