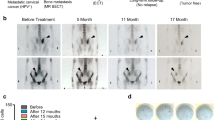
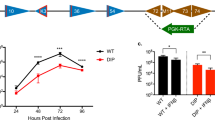
Abstract
Despite promising preclinical results of various therapeutic anticancer immunization strategies, these approaches may not be effective enough to eradicate tumors in cancer patients. While most animal models are based on fast-growing transplantable tumors, malignancies in, for example, cervical cancer patients in general develop much more slowly, which may lead to immune suppression and/or immune tolerance. As a consequence, the immunomodulating signal of any therapeutic immunization regimen should be sufficiently potent to overcome this immunocompromised condition. In previous studies, we demonstrated that an experimental vaccine against human papillomavirus (HPV)-induced cervical cancer, based on Semliki Forest virus (SFV), induces robust HPV-specific cellular immune responses in mice. Now we studied whether this strategy is potent enough to also prime a cellular immune response in immune-tolerant HPV transgenic mice, in which CTL activity cannot be induced using protein or DNA vaccines. We demonstrate that, depending on the route of immunization, SFV-expressing HPV16 E6 and E7 indeed has the capacity to induce HPV16 E7-specific cytotoxic T cells in HPV-transgenic mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walboomers JM et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 89: 12–19.
Munger K, Howley PM . Human papillomavirus immortalization and transformation functions. Virus Res 2002; 89: 213–228.
Eiben GL, Velders MP, Kast WM . The cell-mediated immune response to human papillomavirus-induced cervical cancer: implications for immunotherapy. Adv Cancer Res 2002; 86: 113–148.
Bosch FX et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55: 244–265.
Evans EM, Man S, Evans AS, Borysiewicz LK . Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Res 1997; 57: 2943–2950.
Ressing ME et al. Occasional memory cytotoxic T-cell responses of patients with human papillomavirus type 16-positive cervical lesions against a human leukocyte antigen-A *0201-restricted E7-encoded epitope. Cancer Res 1996; 56: 582–588.
Steinman RM, Nussenzweig MC . Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA 2002; 99: 351–358.
Daemen T, Regts J, Holtrop M, Wilschut J . Immunization strategy against cervical cancer involving an alphavirus vector expressing high levels of a stable fusion protein of human papillomavirus 16 E6 and E7. Gene Therapy 2002; 9: 85–94.
Daemen T et al. Eradication of established HPV16-transformed tumours after immunisation with recombinant Semliki Forest virus expressing a fusion protein of E6 and E7. Vaccine 2003; 21: 1082–1088.
Daemen T et al. Superior therapeutic efficacy of alphavirus-mediated immunization against Human Papilloma virus type 16 antigens in a murine tumor model: effects of the route of immunization. Antiviral Ther 2004; 9: 733–742.
Auewarakul P, Gissmann L, Cid-Arregui A . Targeted expression of the E6 and E7 oncogenes of human papillomavirus type 16 in the epidermis of transgenic mice elicits generalized epidermal hyperplasia involving autocrine factors. Mol Cell Biol 1994; 14: 8250–8258.
Borchers A et al. E7-specific cytotoxic T cell tolerance in HPV-transgenic mice. Arch Virol 1999; 144: 1539–1556.
Michel N et al. Enhanced immunogenicity of HPV 16 E7 fusion proteins in DNA vaccination. Virology 2002; 294: 47–59.
Frazer IH et al. Split tolerance to a viral antigen expressed in thymic epithelium and keratinocytes. Eur J Immunol 1998; 28: 2791–2800.
Lin KY et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996; 56: 21–26.
Feltkamp MC et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 1993; 23: 2242–2249.
van der Burg SH et al. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine 2001; 19: 3652–3660.
Miyahira Y et al. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods 1995; 181: 45–54.
Colmenero P et al. Recombinant Semliki Forest virus vaccine vectors: the route of injection determines the localization of vector RNA and subsequent T cell response. Gene Therapy 2001; 8: 1307–1314.
Barnard P, Payne E, McMillan NA . The human papillomavirus E7 protein is able to inhibit the antiviral and anti-growth functions of interferon-alpha. Virology 2000; 277: 411–419.
Youde SJ et al. Use of fluorogenic histocompatibility leukocyte antigen-A*0201/HPV 16 E7 peptide complexes to isolate rare human cytotoxic T-lymphocyte-recognizing endogenous human papillomavirus antigens. Cancer Res 2000; 60: 365–371.
Tindle RW . Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2: 59–65.
Matzinger P . An innate sense of danger. Semin Immunol 1998; 10: 399–415.
Strauss JH, Strauss EG . The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 1994; 58: 491–562.
Fournier P, Zeng J, Schirrmacher V . Two ways to induce innate immune responses in human PBMCs: paracrine stimulation of IFN-alpha responses by viral protein or dsRNA. Int J Oncol 2003; 23: 673–680.
Wang L et al. Noncoding RNA danger motifs bridge innate and adaptive immunity and are potent adjuvants for vaccination. J Clin Invest 2002; 110: 1175–1184.
Fausch SC, Da Silva DM, Rudolf MP, Kast WM . Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J Immunol 2002; 169: 3242–3249.
Johnston LJ, Halliday GM, King NJ . Phenotypic changes in Langerhans' cells after infection with arboviruses: a role in the immune response to epidermally acquired viral infection? J Virol 1996; 70: 4761–4766.
Glasgow GM et al. The Semliki Forest virus vector induces p53-independent apoptosis. J Gen Virol 1998; 79: 2405–2410.
Acknowledgements
We thank Professor L Gissmann and Dr N Michel from the Deutsches Krebsforschungszentrum, Heidelberg, Germany for kindly providing us with founder transgenic K10HPV16-E6/E7 mice and VP22-E71–60 DNA, and Professor JE Degener from our department for his support and encouragement.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riezebos-Brilman, A., Regts, J., Freyschmidt, EJ. et al. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther 12, 1410–1414 (2005). https://doi.org/10.1038/sj.gt.3302536
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302536
Keywords
This article is cited by
-
Cancer vaccine strategies using self-replicating RNA viral platforms
Cancer Gene Therapy (2023)
-
An alphavirus-based therapeutic cancer vaccine: from design to clinical trial
Cancer Immunology, Immunotherapy (2019)
-
Correlation of Circulating CD64+/CD163+ Monocyte Ratio and stroma/peri-tumoral CD163+ Monocyte Density with Human Papillomavirus Infected Cervical Lesion Severity
Cancer Microenvironment (2017)
-
Perspectives for therapeutic HPV vaccine development
Journal of Biomedical Science (2016)
-
Antigen design enhances the immunogenicity of Semliki Forest virus-based therapeutic human papillomavirus vaccines
Gene Therapy (2015)