Abstract
The variation of δ18O that results from nearly all physical, biological and chemical processes on the Earth is approximately twice as large as the variation of δ17O. This so-called ‘mass-dependent’ fractionation is well documented in terrestrial minerals1,2. Evidence for ‘mass-independent’ fractionation (Δ17O = δ17O - 0.52δ18O), where deviation from this tight relationship occurs, has so far been found only in meteoritic material and a few terrestrial atmospheric substances3. In the rock record it is thought that oxygen isotopes have followed a mass-dependent relationship for at least the past 3.7 billion years (ref. 4), and no exception to this has been encountered for terrestrial solids5. Here, however, we report oxygen-isotope values of two massive sulphate mineral deposits, which formed in surface environments on the Earth but show large isotopic anomalies (Δ17O up to 4.6‰). These massive sulphate deposits are gypcretes from the central Namib Desert and the sulphate-bearing Miocene volcanic ash-beds in North America. The source of this isotope anomaly might be related to sulphur oxidation reactions in the atmosphere and therefore enable tracing of such oxidation. These findings also support the possibility of a chemical origin of variable isotope anomalies on other planets, such as Mars6.
Similar content being viewed by others
Main
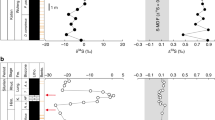
We have analysed sulphates from laboratory experiments and natural sources (Fig. 1). The δ18O and δ17O of seawater sulphate, evaporites, sulphate from microbial sulphate reduction experiments, and sulphates formed from mineral-sulphide oxidation in air or soils, all fall on the mass-dependent terrestrial fractionation line, given by the relationship δ17O = 0.52δ18O. The deviation from this relationship, defined as Δ17O = δ17O - 0.52δ18O, is approximately zero ( -0.04 ± 0.05‰, n = 36). The central Namib Desert gypcretes and the Miocene volcanic ash-falls in the western United States, however, possess sulphate δ18O and δ17O values that deviate from the terrestrial fractionation line. The sulphate Δ17O of Namib gypcretes ranges from 0.20‰ to 0.51‰ (Table 1), well outside the experimental error of ±0.05‰. Gypsum and other water-soluble sulphate minerals from Miocene volcanic ash deposits in Nebraska and South Dakota have strikingly large Δ17O values, up to 4.59‰, in contrast to the zero or slightly positive Δ17O found in adjacent soil and fluvial horizons (Table 2).
a, A fractionation line defined by major sulphate-involving processes on Earth. The seawater sulphate consists of sea water from La Jolla, California, and seawater sulphate adsorbed on the surface of amorphous and poorly crystalline ferric oxides from Red Seamount, Pacific Ocean. Sulphate from the oxidation of mineral-sulphides includes several non-evaporative gypsum minerals from glacial-till deposits in Akron, Ohio, and water-soluble sulphate from surface oxidation of marcasite (FeS2) exposed to air. The sulphate reduction experiment was conducted by adding organic-rich soil into a sealed tank of La Jolla sea water and allowing time for fractionation of oxygen isotopes by microbial sulphate reduction. Gypsum, other sulphate-bearing evaporitic minerals, and sulphate from final residual solution were collected from two seawater evaporation experiments conducted at 22 °C and 55 °C, respectively. b, Gypcrete and gypsum-bearing soils were collected from different soil horizons and different localities in the central Namib Desert. c, Volcanic ash and associated deposits were from the Miocene Arikaree Group, Nebraska and South Dakota (see Table 1 and Table 2 for details). The straight line in b and c is the terrestrial fractionation line (δ17O = 0.52δ18O). The error bar (about ±0.7 for δ18O) was not plotted on the diagrams. Due to the high correlation between δ17O and δ18O during mass spectrometry analysis, the error for Δ17O is very small (less than ±0.05). Thus, a thin rod with slope of 0.52 would best represent the actual point on the diagram. Delta values are given in SMOW.
Sulphate minerals with Δ17O ≈ 0‰ are expected because thermodynamic and kinetic (including biological) processes such as evaporation, mineral-sulphide oxidation (by Fe3+ or air O2), and sulphate reduction generate mass-dependent compositions (Fig. 1). Therefore, sulphate minerals with positive Δ17O values, such as those found in the central Namib Desert gypcretes and the Miocene volcanic ash-falls in the western United States require a different process. The only documented terrestrial reservoirs with positive Δ17O are from the atmosphere. Oxidants such as O3 and H2O2 are known to have positive Δ17O values that range from 1 to more than 25‰ (refs 7,8,9,10). Others, like OH and NOx, remain to be measured.
Tropospheric O3 and H2O2 in rainwater may transfer their positive Δ17O values to the product sulphate by in situ oxidation of surface minerals (for example, sulphides). Our measurement of the sulphate produced by marcasite (FeS2) oxidation in the air yielded Δ17O ≈ 0‰, indicating that the in situ pathway is not the major source of positive Δ17O. A more likely source is the wet and dry atmospheric deposition of sulphate produced by atmospheric oxidation of reduced gaseous sulphur compounds. The anomaly can come from atmospheric oxidants such as O3, H2O2 or OH radicals as a result of aqueous or gas-phase S( IV) oxidation. A similar explanation was invoked to interpret positive Δ17O values (0.20‰ to 1.80‰) observed in aerosol and rainwater sulphate11,12. Although the mechanisms of these atmospheric processes are still subjects of intensive study, the connection between our Δ17O-positive sulphate minerals and atmospheric oxidation processes is unequivocal, as shown by the close association with a high flux of atmospheric reduced sulphur compounds in our two reported cases.
Gypcrete soils in the central Namib Desert occur extensively near the coast and gradually thin off and disappear at about 50–70 km from the coast, constituting one of the most extensive gypsum accumulations in Africa. On the basis of δ34S, meteorological, hydrological, and geological information, Eckardt and Spiro13 suggest that sulphate in the Namib Desert originates mostly from biologically produced marine sulphur (that is, not derived from sea salt), particularly the oxidation of marine dimethyl sulphide (DMS). This conclusion is also supported by the lack of correlation between gypcrete accumulation and bedrock in this location and the positive correlation between gypcrete accumulation and the proximity to the ocean, a source of DMS. The Benguela Current, which originated in the early Late Miocene (about 10 million years ago) off the west coast of South Africa and Namibia, provides upwelled waters extremely rich in phosphate, nitrate and silica, which support a rich population of phytoplankton14 and a high DMS concentration in the local atmosphere. According to this interpretation, the anomalous Δ17O of the Namib gypcretes reflects chemistry associated with atmospheric oxidation of DMS.
Volcanic activity reached its maximum during the Oligocene in western North America15. Layers of volcanic-associated deposits are exposed throughout the Oligocene/Miocene continental deposits in eastern Wyoming, South Dakota, North Dakota and Nebraska16,17. The current water-soluble sulphate content is about 0.02% in the Miocene Rockyford ash-bed (sample BNP-SP). It is well known that volcanic ash contains a significant amount of sulphate, occurring as sulphuric acid droplets that react to form gypsum or other sulphate minerals18. The occurrence of gypsum pseudomorph in the sandy Gering Formation may suggest high sulphate content in overlaid ash-beds. A volcanic origin for the sulphate sulphur is supported by its juvenile δ34S values (Table 2). The large sulphate Δ17O signatures from these widespread Miocene ash-beds indicate a link between the δ17O-anomaly and volcanic processes. Some of the sulphate Δ17O values are more positive than has been observed in sulphate aerosol, rainwater sulphate, and desert gypcrete. We expect the sulphate Δ17O value in volcanic ash will vary depending on distance from source and the nature of eruption.
Sulphate is one of a few non-labile oxygen-bearing ions (aqua-metal ions or oxo-anions)19 that, once produced, are resistant to oxygen exchange with other ambient oxygen-bearing compounds (for example, water) under most surface environments. This chemical feature is essential for the preservation of the anomalous Δ17O signature in sulphate minerals.
The discovery of positive Δ17O values in minerals formed on Earth has important implications in Earth science. First, this is the first, to our knowledge, demonstration of a transfer of δ17O anomaly from atmosphere to crustal minerals. At this time, only a few approaches are available for probing ancient atmospheric conditions20,21,22. Sulphate Δ17O has the potential to be a tracer for ancient atmospheric ozone activity, chemistry in volcanic plumes, and the sulphur biogeochemical cycle in desert environments, where atmospheric sulphate is a major sulphur component. Second, it is interesting to evaluate recent findings of anomalous Δ17O for water, sulphate, and carbonate in SNC meteorites relative to coexisting igneous silicate minerals6,23,24. We document here a larger-magnitude Δ17O signature in terrestrial sulphate minerals than have been observed in SNC meteorites. Our observations are consistent with an atmospheric chemical origin for the SNC oxygen-isotope systematics. To establish the full implications of the δ17O anomalies found in terrestrial minerals, however, requires a fundamental understanding of the sulphur oxidation processes occurring in the atmosphere. The measurements of isotopic fractionation factors for the relevant oxidation reactions will permit quantification of the observations, as has been done for many other species3.
Methods
Barite is precipitated from filtered and acidified solutions, which contain water-soluble sulphate from crushed gypcretes, sand crystals, volcanic ash-beds, and fluvial deposits. Molecular oxygen is extracted directly from barite following a newly developed method25, which employs a 30 W CO2-laser fluorination technique. Fine-grained barite was heated in a BrF5 atmosphere, and more than 90% of the samples were analysed in duplicate. Samples are often loaded and analysed together with other δ17O-normal barites in one set. O2 was run on a Finnigan MAT 251. We found no evidence for mass interference by NF3 fragments or S compounds in our analyses. The samples with large positive Δ17O values were purified and reanalysed using a method26 that quantitatively removes these compounds, after initial mass spectrometric analysis. No differences in Δ17O were observed. The results are highly reproducible and the difference between duplicate analyses is better than ±0.7‰ and ±0.05‰ for δ18O and Δ17O, respectively. Most samples give 28–33% O2 yield during laser fluorination. We found by analysing NBS127 (NIST sulphate standard), seawater sulphate, and a low-δ18O in-house standard that the raw δ18O values are consistently 9.4 lower than the reported ones, owing to the incomplete O2 generation. The correction factor is also verified by more than a dozen natural sulphate samples—that have been analysed using both the laser-fluorination method and the graphite reduction/CO2-fluorination method27—that normally reach a 85% to 100% O2 yield in our laboratory. This comparison also verifies that the incomplete O2-generation using a CO2-laser does not deviate from the slope of 0.52. Thus, the reported δ17O value is increased by 4.89‰. δ34S was analysed using the SF6 method28. In this report, all Δ17O values are calculated on the basis of raw δ17O and δ18O values. We use the linear equation to calculate Δ17O because all the raw δ17O and δ18O values are close to the vicinity of the origin and the range of δ17O and δ18O values among different samples are small.
References
Clayton, R. N., Grossman, L. & Mayeda, T. K. A component of primitive nuclear composition in carbonaceous chondrites. Science 182, 485– 488 (1973).
Matsuhisa, Y., Goldsmith, J. R. & Clayton, R. N. Mechanisms of hydrothermal crystallization of quartz at 250 degrees C and 15 kilobars. Geochim. Cosmochim. Acta 42, 173–182 (1978).
Thiemens, M. H. Atmosphere science—Mass-independent isotope effects in planetary atmospheres and the early solar system. Science 283, 341–345 (1999).
Robert, F., Rejou-Michel, A. & Javoy, M. Oxygen isotropic homogeneity of the Earth: new evidence. Earth Planet. Sci. Lett. 108, 1– 9 (1992).
Clayton, R. N. & Mayeda, T. K. Oxygen isotope studies of achondrites. Geochim. Cosmochim. Acta 60, 1999– 2017 (1996).
Farquhar, J., Thiemens, M. H. & Jackson, T. Atmosphere-surface interactions on Mars: Delta O-17 measurements of carbonate from ALH 84001. Science 280 , 1580–1582 (1998).
Mauersberger, K. Ozone isotope measurements in the stratosphere. Geophys. Res. Lett. 14, 80–83 ( 1987).
Krankowsky, D., Bartecki, F., Klees, G. G., Mauersberger, K. & Schellenbach, K. Measurement of heavy isotope enrichment in tropospheric ozone. Geophys. Res. Lett. 22, 1713–1716 (1995).
Johnston, J. C. & Thiemens, M. H. The isotopic composition of tropospheric ozone in three environments. J. Geophys. Res. 102 (D21), 25395–25404 (1997).
Savarino, J. & Thiemens, M. H. Analytical procedure to determine both delta O-18 and delta O-17 of H2O2 in natural water and first measurements. Atmos. Environ. 33, 3683–3690 (1999).
Lee, C. W. Multiple stable oxygen isotopic studies of atmospheric sulfate aerosols. Am. Geophys. Union 78, F111 ( 1997).
Lee, C. W., Savarino, J. & Thiemens, M. H. Multiple stable oxygen isotopic studies of sulfate and hydrogen peroxide collected from rain water: a new way to investigate in-situ S(IV) oxidation chemistry by dissolved H2O2 in aqueous solution. Am. Geophys. Union 79, F91 (1998).
Eckardt, F. D. & Spiro, B. The origin of sulphur in gypsum and dissolved sulphate in the Central Namib Desert, Namibia. Sedim. Geol. 123, 255–273 (1999).
Siesser, W. G. Late Miocene origin of the Benguela upwelling system off northern Namibia. Science 208, 283–285 (1980).
Armstrong, R. L. & Ward, P. L. Evolving geographic patterns of Cenozoic magmatism in the North American Cordillera; the temporal and spatial association of magmatism and metamorphic core complexes Mid-Tertiary Cordilleran magmatism; plate convergence versus intraplate processes. J. Geophys. Res. B 96, 13201–13224 (1991).
Nicknish, J. M. & Macdonald, J. R. Basal Miocene ash in White River Badlands, South Dakota. Bull. Am. Assoc. Petrol. Geol. 46, 685–690 ( 1962).
Swisher, C. C. III . Stratigraphy And Biostratigraphy Of The Eastern Portion Of Wildcat Ridge, Western Nebraska. Thesis, Univ. Nebraska ( 1982).
Rose, W. I. Jr, Chuan, R. L., Cadle, R. D. & Woods, D. C. Small particles in volcanic eruption clouds. Am. J. Sci. 280, 671–696 (1980).
Gamsjäger, H. & Murmann, R. K. in Advances in Inorganic and Bioinorganic Mechanisms (ed. Sykes, A. G.) 317 –381 (Academic, London, 1983).
Cerling, T. E. Carbon dioxide in the atmosphere – evidence from Cenozoic and Mesozoic paleosols. Am. J. Sci. 291, 377– 400 (1991).
Rye, R. & Holland, H. D. Paleosols and the evolution of atmospheric oxygen: A critical review. Am. J. Sci. 298, 621–672 (1998).
Berner, R. A. et al. Isotope fractionation and atmospheric oxygen: Implications for phanerozoic O2 evolution. Science 287 , 1630–1633 (2000).
Karlsson, H. R., Clayton, R. N., Gibson, E. K. & Mayeda, T. K. Water in SNC meteorites - Evidence for a martian hydrosphere. Science 255, 1409–1411 ( 1992).
Farquhar, J. & Thiemens, M. H. The oxygen cycle of the Martian atmosphere-regolith system: Δ17O of secondary phases in Nakhla and Lafayette. J. Geophys. Res. 105 (E5), 11991–11997 (2000).
Bao, H. & Thiemens, M. H. Generation of O2 from BaSO4 using a CO2-laser fluorination system for simultaneous δ18O and δ17O analysis. Anal. Chem. (in the press).
Clayton, R. N. & Mayeda, T. K. Oxygen isotopes in eucrites, shergottites, nakhlites, and chassignites. Earth Planet. Sci. Lett. 62, 1–6 ( 1983).
Bhattacharya, S. K. & Thiemens, M. H. New evidence for symmetry dependent isotope effects - O+CO reaction. Z. Naturforsch. A J. Phys. Sci. 44, 435–444 (1989).
Forrest, J. & Newman, L. Silver-110 microgram sulfate analysis for the short time resolution of ambient level of sulfur aerosol. Anal. Chem. 49, 1579–1584 (1977).
Acknowledgements
We thank T. Jackson for technical assistance, J. Cannia for sand samples from Scotts Bluff, Nebraska, J. Alt for marine ferric oxide samples, J. Savarino for helpful discussions, and NASA and NSF for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, H., Thiemens, M., Farquhar, J. et al. Anomalous 17O compositions in massive sulphate deposits on the Earth. Nature 406, 176–178 (2000). https://doi.org/10.1038/35018052
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35018052
This article is cited by
-
Sulfate triple-oxygen-isotope evidence confirming oceanic oxygenation 570 million years ago
Nature Communications (2023)
-
The Isotopic Imprint of Life on an Evolving Planet
Space Science Reviews (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.