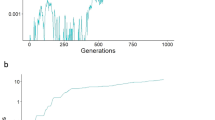
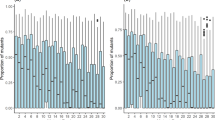
Abstract
How do deleterious mutations interact to affect fitness? The answer to this question has substantial implications for a variety of important problems in population biology, including the evolution of sex1,2,3, the rate of adaptation4,5 and the conservation of small populations3,6,7,8. Here we analyse a mathematical model of competition for food in which deleterious mutations affect competitive ability. We show that, if individuals usually compete in small groups, then competition can easily lead to a type of genetic interaction known as synergistic epistasis. This means that a deleterious mutation is most damaging in a genome that already has many other deleterious mutations. We also show that competition in small groups can produce a large advantage for sexual populations, both in mean fitness and in ability to resist invasion by asexual lineages. One implication of our findings is that experimental efforts to demonstrate synergistic epistasis may not succeed unless the experiments are redesigned to make them much more naturalistic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kondrashov, A. S. Deleterious mutations and the evolution of sexual reproduction. Nature 336, 435–440 ( 1988).
Charlesworth, B. Mutation-selection balance and the evolutionary advantage of sex and recombination. Genet. Res. 55, 199–221 (1990).
Peck, J. R., Barreau, G. & Health, S. C. Imperfect genes, Fisherian mutation and the evolution of sex. Genetics 145, 1171– 1199 (1997).
Charlesworth, B. Directional selection and the evolution of sex and recombination. Genet. Res. 61, 205–224 (1993).
Waxman, D. & Peck, J. R. Sex and adaptation in a changing environment. Genetics 153, 1041– 1053 (1999).
Kondrashov, A. S. Muller's ratchet under epistatic selection. Genetics 136, 1469–1473 (1994).
Kondrashov, A. S. Contamination of the genome by very slightly deleterious mutations—why have we not died 100 times over? J. Theor. Biol. 175 , 583–594 (1995).
Butcher, D. Muller's ratchet, epistasis and mutation effects. Genetics 141, 431–437 (1995).
Kimura, M. & Maruyama, T. The mutational load with epistatic gene interactions in fitness. Genetics 54, 1337–1351 (1966).
Crow, J. F. in Mathematical Topics in Population Genetics (ed. Kojima, K.) (Springer, Berlin, 1970).
de Visser, J. A. G. M., Hoekstra, R. F. & van den Ende, H. An experimental test for synergistic epistasis and its application in Chlamydomonas. Genetics 145, 815–819 (1997).
de Visser, J. A. G. M. & Hoekstra, R. F. Synergistic epistasis between loci affecting fitness: evidence in plants and fungi. Genet. Res. 71, 39–49 ( 1998).
Elena, S. F. & Lenski, R. E. Test of synergistic interactions among deleterious mutations in bacteria. Nature 390 , 395–398 (1997).
Koelewijn, H. P. Effects of different levels of inbreeding on progeny fitness in Plantago coronopus. Evolution 52, 692– 702 (1998).
West, S. A., Peters, A. D. & Barton, N. H. Testing for epistasis between deleterious mutations. Genetics 149, 435–444 (1998).
Willis, J. H. Effects of different levels of inbreeding on fitness components in Mumulus Guttaltus. Evolution 47, 864– 876 (1993).
Eyre-Walker, A. & Keightley, P. D. High genomic deleterious mutation rates in hominids. Nature 397, 344–347 (1999).
Eyre-Walker, A. Evidence of selection on silent site base composition in mammals: Potential implications for the evolution of isochores and junk DNA. Genetics 152, 675–683 ( 1999).
Fry, J. D., Keightley, P. D., Heinsohn, S. L. & Nuzhdin, S. V. New estimates of the rates and effects of mildly deleterious mutation in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 96, 574–579 (1999).
Kondrashov, A. S. & Turelli, M. Deleterious mutations, apparent stabilizing selection and the maintenance of quantitative variation. Genetics 132, 603–618 (1992).
Peck, J. R. & Eyre-Walker, A. The muddle about mutations. Nature 387, 135–136 (1997).
Sved, J. A., Reed, T. E. & Bodmer, W. F. The number of balanced polymorphisms that can be maintained in a natural population. Genetics 55, 469–481 (1967).
King, J. L. Continuously distributed factors affecting fitness. Genetics 55, 483–492 (1967).
Hamilton, W. D., Axelrod, R. & Tanese, R. Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl Acad. Sci. USA 87, 3566–3573 (1990).
Maynard Smith, J. The Evolution of Sex (Cambridge Univ. Press, Cambridge, 1978).
Parker, E. D. in Evolutionary Genetics from Molecules to Morphology (eds Singh, R. & Krimbas, C.) 450–475 (Cambridge Univ. Press, Cambridge, 2000.
Nygren, A. Apomixis in the angiosperms. Bot. Rev. 20, 577–649 (1954).
Bell, G. The Masterpiece of Nature (Univ. California Press, San Francisco, 1982).
Krebs, C. J. Ecology 4th edn (Harper Collins, New York, 1994).
Gradshteyn, I. S. & Ryzhik, I. M. Table of Integrals, Series and Products (Academic, New York, 1980).
Acknowledgements
We thank J. Crow, A. Eyre-Walker, A. Kondrashov and J. Maynard Smith for advice. This study was supported by the Biotechnology and Biological Sciences Research Council (UK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peck, J., Waxman, D. Mutation and sex in a competitive world. Nature 406, 399–404 (2000). https://doi.org/10.1038/35019055
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35019055
This article is cited by
-
The biology of mating in Candida albicans
Nature Reviews Microbiology (2003)
-
Sexual selection and the maintenance of sexual reproduction
Nature (2001)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.