Abstract
β-Catenin is a crucial part of the Wnt and E-cadherin signalling pathways, which are involved in tumorigenesis. Dysregulation of these pathways allow β-catenin to accumulate and translocate to the nucleus, where it may activate oncogenes. Such nuclear accumulation can be detected by immunohistochemistry, which may be useful in diagnosis. Although the role of β-catenin has been established in various types of carcinomas, relatively little is known about its status in mesenchymal tumors. A number of studies suggest that β-catenin dysregulation is important in desmoid-type fibromatosis, as well as in synovial sarcoma. We wished to determine whether nuclear β-catenin expression is specific to and sensitive for particular bone and soft-tissue tumors, including sporadic desmoid-type fibromatosis. We studied the nuclear expression of β-catenin using tissue microarrays in a comprehensive range of bone and soft-tissue tumor types. A total of 549 cases were included in our panel. Nuclear immunohistochemical staining was determined to be either high level (>25% of cells), low level (0–25%) or none. High-level nuclear β-catenin staining was seen in a very limited subset of tumor types, including desmoid-type fibromatosis (71% of cases), solitary fibrous tumor (40%), endometrial stromal sarcoma (40%) and synovial sarcoma (28%). Although occasional cases of fibrosarcoma, clear cell sarcoma and carcinosarcoma had high-level staining, no high-level nuclear β-catenin expression was seen in any of 381 fibrohistocytic, muscular, adipocytic, chondroid or osseous tumor cases representing 42 diagnostic categories. All primary immunostain tissue microarray images are made publicly accessible in a searchable database. High-level nuclear β-catenin staining serves as a useful diagnostic tool, as it is specific to a small subset of mesenchymal tumors.
Similar content being viewed by others
Main
It is known that β-catenin is important in two pathways involved in tumorigenesis.1 First, it interacts with E-cadherin at the cell surface, forming a cadherin–catenin unit. This recruits α-catenin, which in turn binds the intracellular actin cytoskeleton. These interactions have a permissive effect on the formation of intercellular adherens junctions, likely having a role in contact inhibition and in suppressing tumor invasion. However, β-catenin also has a second role in tumor development, where it is a key mediator in the Wnt signalling pathway which regulates cell proliferation and differentiation.2 The levels of free β-catenin in cells are controlled, in the absence of Wnt, by a multiprotein complex that includes adenomatous polyposis coli (APC) and glycogen synthase kinase-3β (GSK3β). This complex promotes the degradation of β-catenin. Activation of the pathway by Wnt leads to an inactivation of GSK3β, which allows β-catenin levels to rise in the cytoplasm, and also to translocate to the nucleus. There, it activates the TCF/LEF transcription factors, which act on a number of Wnt target genes, including c-Myc, tcf1 and cyclinD1. Mutations in this multiprotein complex, most commonly in APC, or in β-catenin itself lead to dysregulated activation of these Wnt target genes, and subsequently result in neoplasia.
The role of the Wnt/β-catenin pathway has been established in a number of tumor types, particularly in the development of colorectal carcinoma and other carcinomas.3 However, there is limited knowledge of its role in bone and soft-tissue tumors. There has been consistent data demonstrating the involvement of this pathway in the pathogenesis of fibromatoses. It is known that dysregulation of β-catenin is important in the development of both an inherited predisposition to fibromatosis, as part of the Gardner syndrome (with familial adenomatous polyposis), and in sporadic desmoid-type fibromatosis.4, 5, 6, 7, 8 Dysregulation of this pathway has also been demonstrated in synovial sarcomas, osteosarcomas,9 liposarcomas and malignant fibrous histiocytomas.10 To demonstrate such dysregulation of this pathway, immunohistochemistry has been used to show abnormal nuclear accumulation of β-catenin. One study has shown that nuclear expression of β-catenin may be used to distinguish mesenteric fibromatosis from gastrointestinal stromal tumors.11 Nuclear localization may also be common in synovial sarcomas12 and high-grade sarcomas with high proliferative activity.13 However, there is little known about β-catenin expression in other mesenchymal tumors.
Therefore, we sought to determine the spectrum of mesenchymal tumors that demonstrate nuclear localization of β-catenin by immunohistochemical analysis of tissue microarrays. Such data would not only have diagnostic value, but could also shed light on the pathogenesis of these disorders.
Materials and methods
Study Cases
Paraffin blocks from bone and soft-tissue tumor resections were retrieved from the archives of Vancouver General Hospital (Vancouver, BC, Canada) and the Stanford Medical Center (Stanford, CA, USA). A single block from each case was selected for use in the construction of the tissue microarray. Tissues from 670 cases were included, representing over 65 diagnostic categories. For the 17 cases of desmoid-type fibromatosis specifically, historical information was available for 15, none of whom had familial adenomatous polyposis. Slides were reviewed and representative areas of tumor were identified on the original blocks by a pathologist with expertise in bone and soft-tissue tumors. The study was approved by the Clinical Research Ethics Board of the University of British Columbia.
Tissue Microarray
A total of four separate tissue microarrays were constructed. The first contains 82 cases of seven types of nonpleomorphic spindle cell tumors, and is designated FH002. Details of this microarray have been published.14 Two other microarrays, designated TA35 and TA38-34, contain 460 cases of 50 types of mesenchymal tumors, including muscle and pleomorphic sarcomas, as described.15 A fourth microarray, designated 03-008, was constructed in the same fashion. This array contains 121 cases of chondroid and osseous tumors, with examples of enchondroma, chondromyxoid fibroma, chondroblastoma, chondrosarcoma (including conventional, mesenchymal, extraskeletal myxoid and dedifferentiated types), osteochodroma and osteosarcoma. Using a tissue microarrayer (Beecher Instruments, Silver Spring, MD, USA), duplicate 0.6 mm cores taken from representative areas from formalin-fixed, paraffin-embedded donor tissue blocks were manually transferred to recipient paraffin blocks. Cores of placental tissue were used as orientation markers.
Immunohistochemistry
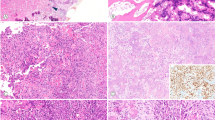
Immunostaining was performed on 4-μm-thick tissue microarray sections, following antigen retrieval by steaming for 40 min in 10 mmol/l citrate buffer (pH 10). Hybridization with the primary β-catenin antibody (clone 14, Transduction Labs, Becton Dickinson, San Diego, CA, USA) was done for 30 min at 1:500 dilution. Detection was made with Envision+ (DAKO Cytomation, Carpinteria, CA, USA), a polymer-based, biotin-free detection system, for 30 min, followed by the chromogen 3,3′-diaminobenzidine for 10 min. The presence of nuclear and cytoplasmic immunostaining was determined semiquantitatively. Nuclear staining was considered positive if at least one tumor cell per high-power field was stained. If greater than 25% of cells were stained, this was scored as ‘high-level’ of staining; less than 25% staining was scored as ‘low-level’ of staining. Cytoplasmic staining was scored in a similar fashion, but was not included in the data analysis. Figure 1 shows typical examples of high- and low-level staining. Where duplicate cores were discrepant, the higher score was used. Cases were not included in analyses if both cores were considered uninterpretable (eg core cut through, absence of tumor cells, absence of viable cells).
Nuclear β-catenin immunostaining in representative cores from the tissue microarrays. High-level immunostaining in: (a) desmoid-type fibromatosis, (b) synovial sarcoma, (c) solitary fibrous tumor, and (d) endometrial stromal sarcoma. (e) Low-level immunostaining in low-grade fibromyxoid sarcoma. (f) Negative nuclear immunostaining (with low-level cytoplasmic immunostaining) in malignant peripheral nerve sheath tumor.
Digital Image Database
We used a BLISS scanner (Bacus Laboratories Inc., Lombard, IL, USA) to acquire digital images from the immunostained and hematoxylin- & eosin-stained tissue microarrays. This automated digital imaging system consists of a microscope with a scanning stage, video camera and software designed for scanning tissue microarrays. A relational database was constructed using identification information and immunohistochemistry scores for each tissue core in the microarrays. An internet website was then constructed using this database and a WebSlideViewer Java applet provided by the manufacturer to view the microarray images and allow for an image zooming functionality. This website is publicly accessible through https://www.gpecimage.ubc.ca/tma/web/viewer.php
Results
A total of 549 tumor cases evaluated for nuclear β-catenin staining were considered interpretable on the tissue microarrays. Table 1 summarizes the results of the analysis. ‘High-level positive’ represents cases with >25% of cells having nuclear staining, ‘Low-Level Positive’ represents cases with 0–25% of cells having nuclear staining, while ‘Total Positive’ represents cases with any nuclear staining. All original images are available for review at https://www.gpecimage.ubc.ca/tma/web/viewer.php and selected examples are presented in Figure 1.
Among the 65 bone and soft-tissue diagnostic entities examined, cases of desmoid-type fibromatosis showed the greatest proportion with high-level nuclear staining (12 of 17 cases). If high-level staining is considered the diagnostic cut-off, this represents a sensitivity of 71%. Within the subset of fibrous tumors, solitary fibrous tumor was the only other tumor type that showed significant high-level staining (six of 15 cases, 40%). Only one out of four cases of fibrosarcoma showed positive staining.
High-level staining was seen in only a limited number of other tumor types. Most significantly, four of 10 (40%) endometrial stromal sarcomas and 16 of 58 (28%) cases of synovial sarcoma had high-level nuclear staining. Otherwise, high-level staining was only found in one of three clear cell sarcomas and three of seven carcinosarcomas (spindle cell carcinomas).
Low-level staining was seen in a variety of tumor types, although infrequently. Most significantly, no cases of malignant fibrous histiocytoma (46 cases), gastrointestinal stromal tumors (37 cases) or osteosarcomas (19 cases), and only rare cases of chondroid, adipocytic, smooth muscle, skeletal muscle and neural tumors showed any β-catenin nuclear staining (Table 1).
In contrast to nuclear staining, cytoplasmic β-catenin staining was seen much more commonly and was therefore present in a much less restricted subset of tumor types. High- and low-level cytoplasmic staining was seen across essentially all subtypes of bone and soft-tissue tumors, and was particularly common in fibroblastic, myofibroblastic and vascular tumors.
Discussion
We conducted this survey of bone and soft-tissue tumors to determine whether nuclear expression of β-catenin, as determined by immunohistochemical staining on tissue microarrays, would be relatively specific to and sensitive for tumors in this class. Among these entities, desmoid-type fibromatosis has the most data supporting a role for β-catenin in its pathogenesis. Our results show that high-level nuclear expression of β-catenin is seen in a very restricted subset of mesenchymal tumors.
A large proportion of cases of desmoid-type fibromatosis do indeed show nuclear immunostaining for β-catenin. This is consistent with a whole section study of 12 cases of desmoid-type fibromatoses, all of which showed >50% nuclear β-catenin expression.16 Recent data has also shown that nuclear β-catenin staining was seen in nearly all cases of mesenteric fibromatosis (nine out of 10 cases).11 Similar data was seen in breast fibromatosis cases, with 27 out of 33 cases (82%) showing >10% staining.17 Another study investigated the immunostaining pattern of β-catenin in fibromatosis, but did not separate nuclear staining from cytoplasmic staining in their data.13 Our results indicate that nuclear β-catenin staining is a sensitive marker for desmoid-type fibromatosis, and when high-level staining is seen, it is quite specific for this tumor type, although a few other tumor types may also show such staining. In particular, solitary fibrous tumors, which share some histologic features, also showed high-level staining. In addition, many synovial sarcoma cases showed nuclear staining, which is similar to reported data where a cut-off of >50% staining was used on whole sections (25 of 44 cases, 57%).12 A notable proportion of endometrial stromal sarcoma and carcinosarcoma (spindle cell carcinoma) cases also showed such staining, which may be relevant in particular differential diagnostic settings. One case of fibrosarcoma and one case of clear cell sarcoma did show high-level staining, but since our panel included only three cases of each tumor type, further studies are needed to investigate β-catenin in these two sarcomas.
Our findings in endometrial stromal sarcomas (ESS) are interesting, as there is no data regarding β-catenin immunohistochemistry in ESS in the literature. These findings are potentially quite useful as ESS may pose a diagnostic challenge, particularly in distinguishing from smooth muscle tumors.18 As no cases of leiomyoma (out of eight) and only one out of 41 cases of leiomyosarcoma showed any nuclear staining for β-catenin in our panel, β-catenin appears quite specific for ESS when looking at these tumor types, and is relatively sensitive (60%, when both high- and low-level staining is considered).
No other tumor type showed any high-level nuclear staining, and only a small proportion had low-level staining, which is highly consistent with those previous reports that are available. In one study, looking at 11 cases of gastrointestinal stromal tumors, there were no cases with nuclear β-catenin staining.11 Another study looked at 18 cases of well-differentiated liposarcoma, 12 cases of dedifferentiated liposarcoma and nine cases of malignant fibrous histiocytoma, none of which showed ‘positive’ nuclear staining;10 these results are very consistent with our findings. One study looking at osteosarcoma reported 33 of 47 cases to have cytoplasmic and/or nuclear β-catenin staining,9 whereas our data showed no nuclear staining in 19 cases. However, only three of these 47 cases in fact had nuclear staining (Cheng H and Haydon RC, personal communication), and this was seen when using a higher primary antibody concentration and longer incubation time.
As nuclear accumulation of β-catenin suggests activation of the Wnt signalling pathway, possibly due to dysregulation of β-catenin levels, it is reasonable to suggest that this pathway may be involved in the pathogenesis of the tumor types showing significant nuclear staining. Numerous studies support this notion for desmoid-type fibromatosis. There have been consistent observations in cases of desmoid-type fibromatoses that mutations are frequently seen in proteins regulating β-catenin (especially APC) or in β-catenin itself, which leads to activation of oncogenes downstream in the pathway. Our results certainly support these findings. A significant number of cases of solitary fibrous tumors also showed nuclear β-catenin. There are no current data linking β-catenin to the pathogenesis of solitary fibrous tumors. However, the Wnt/β-catenin pathway has been linked to fibroproliferative disorders more generally in animal studies, in particular hyperplastic cutaneous wounds.19 Furthermore, our data relating to synovial sarcoma is consistent with published reports, as APC mutations have been observed,20 and β-catenin mutations and nuclear accumulation have been correlated with poor prognosis in this tumor type.12, 21
Tissue microarray technology allows high throughput immunohistochemical analysis of archival material, with parallel processing of specimens, and is increasingly being used in pathology investigations. Using duplicate cores helps reduce sampling errors,22, 23 but the extent of focal staining might still be underestimated using this technique. Another difficulty in extrapolating this study for diagnosis is the criteria used to define ‘positive’ and ‘negative’ staining, and for this reason, the primary image data and assigned scores are included in the companion web site. We used high-level (>25% of cells) staining, rather than any positive staining (>0%), as the cut-off in our analysis; nevertheless, changing this cut-off value would not significantly alter the major findings of this study. This cut-off appears to be optimal for diagnostic purposes by maximizing the number of cases of desmoid-type fibromatosis considered truly positive. Among the other mesenchymal tumor types in this study, 23 of 451 cases (5.1%) demonstrated low-level positivity. Lastly, future studies could improve on the low case numbers for certain tumor types in our panel, especially various other types of fibrous tumors.
In summary, high-level nuclear β-catenin staining may be useful as a diagnostic marker for desmoid-type fibromatosis, as only a very limited subset (solitary fibrous tumor, synovial sarcoma, and endometrial stromal sarcoma) of other mesenchymal neoplasms are positive for this marker. These data also support previous studies noting a role for the Wnt/β-catenin pathway in desmoid-type fibromatosis and in synovial sarcoma, and may suggest a role in endometrial stromal sarcoma and solitary fibrous tumor.
References
Ilyas M, Tomlinson IP . The interactions of APC, E-cadherin and beta-catenin in tumour development and progression. J Pathol 1997;182:128–137.
van Es JH, Barker N, Clevers H . You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev 2003;13:28–33.
Polakis P . The oncogenic activation of beta-catenin. Curr Opin Genet Dev 1999;9:15–21.
Tejpar S, Li C, Yu C, et al. Tcf-3 expression and beta-catenin mediated transcriptional activation in aggressive fibromatosis (desmoid tumour). Br J Cancer 2001;85:98–101.
Saito T, Oda Y, Tanaka K, et al. Beta-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumours. J Pathol 2001;195:222–228.
Tejpar S, Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999;18:6615–6620.
Miyoshi Y, Iwao K, Nawa G, et al. Frequent mutations in the beta-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res 1998;10:591–594.
Alman BA, Li C, Pajerski ME, et al. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997;151:329–334.
Haydon RC, Deyrup A, Ishikawa A, et al. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer 2002;102:338–342.
Sakamoto A, Oda Y, Adachi T, et al. Beta-catenin accumulation and gene mutation in exon 3 in dedifferentiated liposarcoma and malignant fibrous histiocytoma. Arch Pathol Lab Med 2002;126:1071–1078.
Montgomery E, Torbenson MS, Kaushal M, et al. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol 2002;26:1296–1301.
Hasegawa T, Yokoyama R, Matsuno Y, et al. Prognostic significance of histologic grade and nuclear expression of beta-catenin in synovial sarcoma. Hum Pathol 2001;32:257–263.
Kuhnen C, Herter P, Muller O, et al. Beta-catenin in soft-tissue sarcomas: expression is related to proliferative activity in high-grade sarcomas. Mod Pathol 2000;13:1005–1013.
Nielsen TO, Hsu FD, O'Connell JX, et al. Tissue microarray validation of epidermal growth factor receptor and SALL2 in synovial sarcoma with comparison to tumors of similar histology. Am J Pathol 2003;163:1449–1456.
West RB, Harvell J, Linn SC, et al. APO D in soft-tissue tumors: a novel marker for dermatofibrosarcoma protuberans. Am J Surg Pathol 2004, [abstract].
Saito T, Oda Y, Kawaguchi K, et al. Possible association between higher beta-catenin mRNA expression and mutated beta-catenin in sporadic desmoid tumors: real-time semiquantitative assay by TaqMan polymerase chain reaction. Lab Invest 2002;82:97–103.
Abraham SC, Reynolds C, Lee JH, et al. Fibromatosis of the breast and mutations involving the APC/beta-catenin pathway. Hum Pathol 2002;33:39–46.
Amant F, Moerman P, Cadron I, et al. The diagnostic problem of endometrial stromal sarcoma: report on six cases. Gynecol Oncol 2003;90:37–43.
Cheon SS, Cheah AY, Turley S, et al. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA 2002;99:6973–6978.
Saito T, Oda Y, Sakamoto A, et al. APC mutations in synovial sarcoma. J Pathol 2002;196:445–449.
Saito T, Oda Y, Sakamoto A, et al. Prognostic value of the preserved expression of the E-cadherin and catenin families of adhesion molecules and of beta-catenin mutations in synovial sarcoma. J Pathol 2000;192:342–350.
Hoos A, Urist MJ, Stojadinovic A, et al. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 2001;158:1245–1251.
Camp RL, Charette LA, Rimm DL . Validation of tissue microarray technology in breast carcinoma. Lab Invest 2000;80:1943–1949.
Acknowledgements
This research is supported by The Terry Fox Foundation. TO Nielsen is a Scholar of the Michael Smith Foundation for Health Research. The automated digital imaging system for tissue microarrays was purchased with a grant from the Canada Foundation for Innovation. MCU Cheang is supported by an educational grant from Aventis Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Duality of interest
None declared.
Rights and permissions
About this article
Cite this article
Ng, T., Gown, A., Barry, T. et al. Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 18, 68–74 (2005). https://doi.org/10.1038/modpathol.3800272
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800272
Keywords
This article is cited by
-
Case report: pseudoendocrine sarcoma, a clinicopathologic report of a newly described soft tissue neoplasm
Virchows Archiv (2023)
-
Efficacy of auranofin as an inhibitor of desmoid progression
Scientific Reports (2022)
-
Usefulness of β-catenin expression in the differential diagnosis of osteosarcoma, osteoblastoma, and chondroblastoma
Virchows Archiv (2021)
-
Synovial Sarcoma: A Complex Disease with Multifaceted Signaling and Epigenetic Landscapes
Current Oncology Reports (2020)
-
Intra-articular nodular fasciitis: a rare lesion case report and an updated review of the literature
BMC Musculoskeletal Disorders (2019)