Abstract
Our recent investigations showed that polycomb chromobox 4 (Cbx4) promotes angiogenesis and metastasis of hepatocellular carcinoma (HCC) through its sumoylating action on hypoxia-inducible factor-1α protein. Here, we attempt to identify the prognostic significances of Cbx4 by a retrospective analyses in 727 cases of HCC patients with and without postoperative transarterial chemoembolization (TACE) or transarterial embolization (TAE). Binary logistic regression tests indicated that Cbx4 is correlated with histological grading, tumor-node-metastasis stage, microvessel density, distant metastasis and hematogenous metastasis of HCC. By univariate and multivariate analyses, we show that Cbx4 is an independent prognostic factor of HCC, and both TAE and TACE treatments have no effects on the overall survival in HCC patients with low Cbx4 expression. More intriguingly, TACE prolongs, while TAE shortens, the overall survival of HCC patients with high Cbx4 expression, indicating that Cbx4 is a good biomarker on decision-making to perform postoperative TACE in HCC patients. Moreover, Cbx4 overexpression enhances while Cbx4 silencing antagonizes doxorubicin-induced cell death of HCC cell lines. In conclusion, Cbx4 is an independent prognostic factor for HCC patients, and the patients with high Cbx4 expression should receive postoperative TACE treatment to improve their survival.
Similar content being viewed by others
Main
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh in women. Because of its high fatality, it has been regarded as the third most common cause of death from cancers worldwide.1, 2 To date, surgery remains the only curative modality for HCC. However, long-term prognosis of HCC patients who underwent curative hepatic resection is still far from settled.3 In the past years, there has been an explosion in our understanding of the molecular alternations occurring in HCC, through which a series of molecular biomarkers were identified in terms of both their prognostic significances and potentials as therapeutic targets.4, 5, 6, 7, 8, 9 It is probable that malignant angiogenesis is among the strongest signals for aberrant pathway activation in HCC, a hypervascular tumor.10 Thus, angiogenic cytokines such as vascular endothelial growth factor (VEGF) may play crucial roles in the pathogenesis of HCC. Based on these discoveries, anti-angiogenesis therapies including drugs targeting VEGF, such as sorafenib, has been approved to be used as the first systemic therapy of HCC, marking a major milestone in the treatment of advanced HCC. However, anti-angiogenic agents have not been as successful as initially imagined in HCC, mainly because tumors that typically responded initially to anti-VEGF therapies quickly become resistant.10, 11, 12, 13 However, transarterial embolization (TAE) and transarterial chemoembolization (TACE) have become the major interventional treatments for HCC patients. Current guidelines recommend that curative hepatic resection is indicated only in patients with early-stage HCC and satisfactory liver function. In contrast, TACE is recommended as the standard treatment of intermediate-stage HCC in Barcelona-Clinic Liver Cancer (BCLC) algorithm.14, 15 Although it was reported that TACE was more effective in 3- to 5-cm tumors than in smaller ones,16 the long-term survival outcomes of patients managed with TACE do not appear fully satisfactory.13, 17 A meta-analysis with low risk of section of bias showed that TACE or TAE did not significantly increase survival of intermediated-stage HCC patients.18 Hepatic resection is considered as the most effective treatment for HCC patients,19, 20, 21 while TAE/TACE is often performed as a postoperative adjuvant therapy to improve the survival of patients.22, 23, 24 However, the efficacy and safety of postoperative TAE/TACE is poorly understood. Meanwhile, because HCC includes a heterogeneous population of patients with varying tumor burdens,3, 25 it is important to discern what kinds of HCC and genetic profiles can benefits from postoperative TAE or TACE treatment.
Polycomb chromobox homolog (Cbx) protein, which includes different isoforms such as Cbx2, Cbx4, Cbx6, Cbx7 and Cbx8, is a critical component of polycomb repressive complex 1 (PRC1), which acts together with PRC2 to silence gene expressions by specifically modifying nucleosomal histones.26, 27, 28, 29 Besides functioning in PRC1-mediated transcription repression, Cbx4 (also known as polycomb 2, Pc2) is also a SUMO (small ubiquitin-related modifier) E3 ligase,30 which can enhance the sumoylation of a more limited repertoire of substrates involved in tumorigenesis.31 In an early oligonucleotide microarray-based transcription profile analysis including 50 hepatocellular nodular lesions ranging from low-grade dysplastic nodules to primary HCC, Cbx4 was shown to be among the top clusters of genes that were highly correlated with ostensible biological implications.32 More recently, Cbx4 expression was reported to be upregulated in cancer tissues and high Cbx4 expression was correlated with α-fetoprotein (AFP) level in serum, tumor size, pathologic differentiation, and TNM (tumor, node, metastasis) stages based on the analysis of 246 cases of HCC specimens.33 More intriguingly, our group reported that Cbx4 enhances sumoylations of oxygen-sensitive hypoxia-inducible factor 1 alpha (HIF-1α), governing its transcriptional activity,34 through which Cbx4 increases VEGF production and promotes angiogenesis and metastasis in vitro and in vivo in HCC.34, 35, 36 Accordingly, Cbx4 expression has a significant positive correlation with VEGF expression in a cohort with 727 cases of HCC specimen.34 Herein, we continue to investigate the prognostic significance of Cbx4 expression in this cohort of HCC patients with or without postoperative adjuvant TAE and/or TACE treatment.
Results
Univariate analyses identify Cbx4 expression as a significant prognostic predictor for survival of HCC patients
Although we and others previously showed that high Cbx4 expression was significantly correlated with poor overall survival (OS) of HCC patients,33, 34 it remains to be addressed whether Cbx4 expression is an independent prognostic factor for HCC patients. For this purpose, we performed the univariate analyses to test the relationships between relatively expressed Cbx4 levels by immunohistochemistry (IHC) as described previously34 and standard variables to OS or disease-free survival (DFS) in our previously reported cohort of 727 cases of HCC patients. Of note, IHC scores were made for all Cbx4 proteins, which were detectable in both nucleus and cytoplasm in HCC tumor tissues. As shown in Table 1, genders, nationalities, HBsAg, anti-HCV, cirrhosis, drinking and smoking status, tumor number and AFP levels were not associated with survival, while Cbx4 expression (P=5.4 × 10−9 for OS, P=0.001 for DFS), microvessel density (MVD; P=5.6 × 10−5 for OS, P=0.028 for DFS) and histological grade (P=2.6 × 10−6 for OS, P=0.041 for DFS) were among the significant prognostic factors for both OS and DFS in HCC patients. Notably, tumor size and TNM stage were only associated with OS (P=0.0002 and P=0.001, respectively).
Cbx4 expression is positively correlated with histological grading and metastasis of HCC
Next, we assessed the potential relationship between Cbx4 expression and standard variables of HCC patients by χ2 test. The results showed that Cbx4 expression was positively correlated with histological grading, MVD and TNM stage as well as distant metastasis and hematogenous metastasis (HM), two HCC features obtained from the end of follow-up interviews or the last follow-up interview before death (Table 2). Cancer tissues with high histological grade (Figure 1a) presented high scores of Cbx4 staining, and cancer tissues with high Cbx4 expression presented more vascular invasion (Figure 1a). Moreover, higher Cbx4 expression increased the undifferential risk of HCC with an odds ratio (OR) of 1.365 (95% CI 1.008–1.849, P=0.044; Figure 1b), high TNM risk (OR 2.191, 95% CI 1.505–3.190, P=3.3 × 10-5; Figure 1c), HM risk (OR 1.797, 95% CI 1.256–2.573, P=0.001; Figure 1d), and distant metastasis risk (OR 2.349, 95% CI 1.001–5.454, P=0.041; Figure 1e) besides MVD reported previously (OR 11.706, 95% CI 8.170−16.772, P=4.3 × 10−47; Figure 1f).
Cbx4 expression is positively correlated with histological grading and metastasis of HCC. (a) The representative images for Cbx4 expression and H&E staining in tumors with low and high grade and with or without vascular invasion. (b–f) The undifferential risk (b), high TNM stage (c), HM+ risk (d), distant metastasis risk+ (e) and high MVD risk (f) were compared between tumors with high and low Cbx4 expression in 727 cases of HCC patients. Corresponding risk value odds ratio is calculated using binary logistical regression with likelihood-ratio test for forward method
Cbx4 expression is an independent prognostic factor for HCC patients
Because high grade, TNM stage and high MVD were associated with poor clinical outcome, we aimed to determine whether reduced OS and DFS observed in patients with high expression of Cbx4 was an indirect reflection of association between higher Cbx4 expression and these clinicopathological markers or, alternately, whether higher Cbx4 expression might be an independent prognostic factor. To answer this, multivariate analysis based upon Cox proportional hazard regression model was performed. Tumor size (HR 1.875, 95% CI 1.382–2.545, P=5.4 × 10−5), histological grade (HR 1.588, 95% CI 1.256–2.008, P=1.1 × 10-4) as well as Cbx4 expression (HR 1.705, 95% CI 1.301–2.233, P=1.0 × 10−4) were determined as independent prognostic factors for OS. For DFS, histological grade (HR 1.432, 95% CI 1.034–1.983, P=0.031) and Cbx4 expression (HR 1.493, 95% CI 1.027–2.170, P=0.036) were determined as independent prognostic factors (Table 3). Further stratified analyses based on histological grade and tumor size were performed. In low-grade tumors, high Cbx4 expression had poorer OS (P=2.5 × 10−6; Figure 2a, left) and DFS (P=4.0 × 10−4; Figure 2a, right), and Cbx4 expression also presented an inverse correlation with OS outcomes of patients with high-grade tumors (P=3.0 × 10−4; Figure 2b, left), although no significant statistical difference was found for DFS in high-grade patients with tumors of low and high Cbx4 expression (Figure 2b, right). Also, tumors with high Cbx4 expression had poorer DFS and OS regardless of whether the tumor size was bigger or smaller than 5 cm (Figures 2c and d).
High Cbx4 expression differentially affects therapeutic effect of TAE and TACE intervention in HCC patients
Based on the fact that highly vascularized tumors are supplied by the hepatic arteries whereas the non-neoplastic liver parenchyma has dual blood supply,37 TAE and TACE have gained considerable attentions and been advocated as standard locoregional life-extending treatment for unresectable HCC or as adjuvant therapy after surgery to improve HCC patients’ survival.38 However, Cbx4 can enhance hypoxia-driven VEGF expression and angiogenesis as a SUMO E3 ligase on HIF-1α protein in HCC cells.34 Thus, we asked whether Cbx4 and VEGF expression levels impinge on the therapeutic effects of TAE or TACE intervention as adjuvant therapy after surgery in HCC patients. The stratified analysis based on Cbx4 or VEGF expression level showed that TAE and TACE treatment had no effect on the OS of patients with HCC tumors of low Cbx4 or VEGF expression (Figure 3a). More intriguingly, in patients with HCC tumors of high Cbx4 or VEGF expression, TACE prolonged whereas TAE treatment significantly shortened their OS (Figure 3b). In line with this notion, TAE/TACE did not affect the risk of death in patients with HCC tumors of low Cbx4 or VEGF expression (Figure 3c). TACE treatment decreased the risk of death in HCC patients with high Cbx4 expression (TACE versus control, HR 0.596, 95% CI 0.410–0.868; P=0.007) or high VEGF expression (TACE versus control, HR 0.690, 95% CI 0.491–0.969; P=0.032), while TAE increased the risk of death in patients with high Cbx4 expression (TAE versus control, HR 1.533, 95% CI 1.114–2.110; P=0.009) or high VEGF expression (TAE versus control, HR 1.396, 95% CI 1.017–1.918; P=0.039; Figure 3d). By using control group with low Cbx4/VEGF expression as a reference, further, a joint statistical analysis between Cbx4 or VEGF expression and TAE/TACE treatment on HCC prognosis was performed. The results showed that the cases with HCC tumors of high Cbx4 or VEGF expression would face a decreased risk of death with TACE treatment. However, an increased risk of death was associated with TAE treatment (Figure 3e). These results suggest that Cbx4 as well as VEGF expressions should be able to modify the effects of TAE/TACE treatment to predict the survival of HCC patients.
TAE shortens while TACE prolongs the survival of patients with tumors of high Cbx4 or high VEGF expression. (a and b) Comparison of overall survival among control, TAE and TACE treatment groups of patients with low (a) and high Cbx4 or VEGF expression (b). The P-values for three groups of patients are shown. (c and d) Comparison of relative risks of death among the indicated three groups of patients with low (c) and high Cbx4 or VEGF expression (d). The P-values for three groups of patients are shown. (e) Joint analyses between Cbx4 or VEGF expression and TAE/TACE treatment. The P-values for six groups of patients are shown
Cbx4 overexpression increases doxorubicin-induced death of HCC cells
Based on the above-mentioned findings that TACE was beneficial while TAE was harmful to HCC patients with tumors of high Cbx4 expression, we hypothesize that Cbx4 can affect the sensitivity of HCC cells to chemotherapeutic drugs. To address this, two HCC cell lines, SMMC-7721 and MHCC97L, were stably infected with retrovirus carrying Flag alone or Flag-tagged Cbx4 (Figures 4a and b), followed by the treatment with increasing concentrations (from 0.1 to 20 μM for SMMC-7721 (Shanghai, China) and from 0.1 to 40 μM for MHCC97L (Shanghai, China)) of doxorubicin, a commonly used drug in TACE intervention. Thirty-six hours later, CCK-8 assay showed that Cbx4 overexpression significantly increased sensitivity of both HCC cell lines to doxorubicin (Figures 4c and d). The half-maximal inhibitory concentrations (IC50) of doxorubicin were 0.629 versus 1.244 μM and 3.414 versus 5.969 μM in Flag-tagged Cbx4 and empty vector-infected SMMC-7721 and MHCC97L cells, respectively. Furthermore, Cbx4 overexpression significantly increased doxorubicin-induced cell death in these two cell lines, as assessed by TUNEL assay (Figures 4e and f) and proteolytic activations of caspases 9 and 3 as well as poly(ADP-ribose)polymerase (PARP) cleavage (Figures 4a and b).
Cbx4 increases the sensitivity of SMMC-7721 and MHCC97L cells to doxorubicin-induced cell death. SMMC-7721 and MHCC97L cells were stably infected with empty or Flag-tagged Cbx4. (a and b) Doxorubicin was added into SMMC-7721, 2 μM (a) and MHCC97L, 3 μM (b) cells for 36 and 48 h, followed by immunoblots for the indicated proteins. (c and d) SMMC-7721 (c) and MHCC97L (d) with or without ectopic Cbx4 expression were respectively treated with and without different concentrations of doxorubicin for 36 h, and cell growth was assessed by CCK-8 assay and IC50 values of doxorubicin were calculated by GraphPad Prism 6 software. (e and f) SMMC-7721 (e) and MHCC97L (f) with or without ectopic Cbx4 expression were treated with 2 μM (e) or 3 μM (f) of doxorubicin for 36 and 48 h, and percentages of TUNEL-positive cells were calculated
Cbx4 knockdown antagonizes doxorubicin-induced death of HCC cells
Finally, we also knocked down Cbx4 expression in SMMC-7721 and MHCC97L cells using two pairs of shRNAs specifically against Cbx4 (shCbx4#4 and shCbx4#5) together with non-specific shRNA as a negative control (NS). As depicted in Figures 5a and b, the two specific shRNAs could effectively silence Cbx4 expression in these two cell lines. Contrary to what was seen in Cbx4-overexpressed HCC cells, Cbx4 silencing remarkably increased IC50 of doxorubicin in SMMC-7721 cells (Figure 5c), and especially in MHCC97L cells (Figure 5d). Accordingly, Cbx4 knockdown also inhibited doxorubicin-induced cell death, as assessed by TUNEL assay (Figures 5e and f) and cleaved activations of caspases 9 and 3 as well as PARP cleavage (Figures 5a and b). Note that ectopically expressed Cbx4 (Figures 4a and b) and endogenously expressed Cbx4 protein (Figures 5a and b) were decreased during cell death induction.
Cbx4 knockdown inhibits the cell death sensitivity of SMMC-7721 and MHCC97L upon doxorubicin treatment. SMMC-7721 and MHCC97L cells were stably infected with two pairs of shRNAs specifically against Cbx4 (shCbx4#4 and shCbx4#5) together with non-specific shRNA as a negative control (NS). (a and b) Doxorubicin was added into SMMC-7721, 2 μM (a) and MHCC97L, 3 μM (b) cells for 36 and 48 h, followed by immunoblots for the indicated proteins. (c and d) SMMC-7721 (c) and MHCC97L (d) were respectively treated with and without different concentrations of doxorubicin for 36 h, and cell growth was assessed by CCK-8 assay and IC50 values of doxorubicin were calculated by GraphPad Prism 6 software. (e and f) SMMC-7721 (e) and MHCC97L (f) with or without Cbx4 knockdown were treated with 2 μM (e) or 3 μM (f) of doxorubicin for 36 and 48 h, and percentages of TUNEL-positive cells were calculated
Discussion
Here we reported that either TAE or TACE could not improve the survival of HCC patients with low Cbx4 or VEGF expression. However, TAE and TACE presented different effects on the survival of HCC patients with high Cbx4 or VEGF expression. On one hand, TAE treatment shortened the survival of these HCC patients, consistent with the fact that TAE induced ischemic conditions under which Cbx4 enhanced transcriptional activity of HIF-1, promoting the progression of HCC.34, 39, 40 On the other hand, TACE treatment could significantly improve the survival of HCC patients with high Cbx4/VEGF expression. All these data suggest that Cbx4 or VEGF can act as an indicator for TACE treatment of HCC patients.
The effects of Cbx4 on tumorigenesis are very complicated. It not only acts as a critical component of PRC1 to contribute to the repression of gene expression by epigenetic modification on chromatin, but also functions as a SUMO E3 ligase to regulate tumorigenesis- or DNA-damage-associated proteins, such as heterogeneous nuclear ribonucleoprotein (hnRNP) K. As reported,41 sumoylation of hnRNP K was regulated by the E3 ligase activity of Cbx4, through which DNA damage stimulated hnRNP K sumoylation. The difference of Cbx4 expression on the therapeutic effects of TACE prompted us to investigate why Cbx4 increases the therapeutic benefit of TACE, but not that of TAE, in HCC patients. More intriguingly, we showed that Cbx4 overexpression significantly enhanced, while Cbx4 silencing antagonized, doxorubicin-induced death of HCC cells, supporting the notion that Cbx4 increases the sensitivity of HCC cells to doxorubicin in TACE treatment. The mechanisms underlying Cbx4-increased cell death remain to be further investigated.
In conclusion, our results propose that Cbx4 can act as a biomarker for the prognosis of HCC patients and for predicting the therapeutic effectiveness of TACE on these patients. Furthermore, Cbx4-increased sensitivity of HCC cells to chemotherapeutic drugs together with the Cbx4-increased hypoxia-stimulated angiogenesis34, 36 supported the notion that VEGF inhibitor sorafenib in combination with TACE improves the survival of patients,37, 42 although more analysis (including prospective study and pathogenesis analysis) deserve further elucidation based on a large sample.
Materials and Methods
Patients and specimens
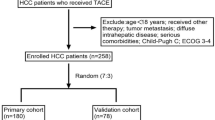
We obtained formalin-fixed and paraffin-embedded tumor specimens of HCC patients, who were histopathologically diagnosed between January 2004 and December 2010 in the Department of Pathology, Affiliated Hospitals of Youjiang Medical College for Nationalities and Guangxi Medical University, as previously described.34 All tumors were primary and were untreated before hepatic resection. Retrospective analyses were performed on clinicopathologic data of these 727 HCC patients who underwent curative hepatectomy in the hospital. Of the 727 patients, 186 (25.6%) received postoperative TAE, 154 (21.2%) underwent postoperative TACE, according to Chinese Manage Criteria of HCC,43 and the rest (387, 53.2%) received neither after hepatic resection according to the patients’ will. The corresponding survival status was confirmed by patients or family contacts. After obtaining written consent, demographic and clinical data were collected in the hospital using a standard interviewer-administered questionnaire and/or medical records. Follow-up interviews ended either by death or on 31 October 2011.
HCC was diagnosed based on evidence of the combination of hepatic angiography, enhanced CT and/or MRI and pathology. Recurrence status was diagnosed by imaging techniques, either intrahepatically or extrahepatically (lymph nodes, distant metastasis). An increased AFP without radiologic evidence was not diagnosed as recurrence until it manifested on imaging. Removed samples of all cases were collected for analyzing the protein expression levels of Cbx4, VEGF and CD31, and H&E staining for pathological analysis was conducted. For histologically differentiated degree of cancer tissues, patients were divided into well and moderately differentiated (low grade), poorly differentiated and undifferentiated (high grade) groups. Classification of TNM stage was assessed according to the sixth edition of the TNM classification system of the American Joint Committee on Cancer/International Union Against Cancer.
To evaluate the role of adjuvant treatment of postoperative TAE/TACE on hepatectomy in patients with low or high Cbx4 expression, all corresponding TAE/TACE treatment information was collected. In this study, TACE consisted of an injection containing a mixture of chemotherapeutic agents and lipiodol followed by embolization with gelatin foam or polyvinyl alcohol until complete stasis was achieved in the tumor-feeding vessels. The chemotherapeutic agents used concurrently included epirubicin, cisplatin and fluorouracil (5-FU). TAE was performed following the same process without chemotherapeutic treatment. According to whether the HCC patients received TAE/TACE treatment, all cases were divided into three groups: (1) TACE, (2) TAE and (3) control groups.
Immunohistochemistry staining
The protein levels of Cbx4 and VEGF in cells of tumor tissues were analyzed by IHC, respectively with anti-Cbx4 antibody (1 : 25 dilution, SC-19299, Santa Cruz Biotechnology, Dallas, TX, USA) and anti-VEGF polyclonal antibody (1 : 500 dilution, SC-152, Santa Cruz Biotechnology). Angiogenesis was assessed using IHC with anti-CD31 antibody (1 : 50 dilution, Gene Tech, Shanghai, China). All primary antibody stainings were followed by staining with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (KIT-9719 and KIT-9707, Maixin Biotechnology, Fuzhou, China). IHC scores of Cbx4 and VEGF were divided into two classifications: low (immunoreactive scores ≤4) and high (immunoreactive scores >4), according to the value of IRS systems as described in our previous work.34, 42 At × 200 magnification, vessel count was made of all distinct brown-staining endothelial cells in the cancerous regions over five fields in each slide. MVD was defined as the average value of three readings. Angiogenesis status was assessed by MVD classification: low (≤50/ × 200 magnification) and high (>50/ × 200 magnification), according to the mean MVD of cancerous-tissue vessels. Images were captured with Nikon Ti-S microscope equipped with a digital camera system (Nikon, Tokyo, Japan).
Cell culture and transfection
SMMC-7721 were obtained from Cell Resource Center of Shanghai Institute for Biological Sciences, CAS, Shanghai, China. MHCC97L was kindly provided by Dr. Lunxiu Qing at Fudan University (Shanghai, China). These two cell lines were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1% penicillin and streptomycin, supplemented with 10% fetal bovine serum (FBS). Flag-tagged Cbx4, which came from a Cbx4-expressing pCMV plasmid with amino-terminal Flag-tag, generously provided by Dr. Weng JM at East China Normal University (Shanghai, China), was cloned into a pBABE purovector (Cell Biolabs, Inc., San Diego, CA, USA) for stable transfection, as described previously.34 Retroviruses were prepared by transient co-transfection with a helper plasmid into HEK293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following manufacturer’s protocol.
Western blots
Protein extracts were separated and quantified by 10 and 12.5% SDS-polyacrylamide gel, and transferred to the Immobilon PVDF transfer membranes (Millipore Corporation, Boston, MA, USA). After blocking with 5% nonfat milk in PBS, membranes were immunoblotted with anti-Flag (F1804, Sigma-Aldrich, St. Louis, MO, USA), anti-caspase 3 (#9662, Cell Signaling Technology, Billerica, MA, USA), anti-caspase 9 (#9502, Cell Signaling Technology), anti-PARP-1 (sc-8007, Santa Cruz Biotechnology), anti-Cbx4 (sc-19299, Santa Cruz Biotechnology) antibodies, and then with HRP-conjugated secondary antibody (Cell Signaling Technology) for 2 h at room temperature. Anti-α-tubulin-HRP-conjugated antibody (PM054-7, MBL, Woburn, MA, USA) acted as the internal control. Signals were detected by Immobilon Western chemiluminescent HRP substrate (Millipore Corporation).
TUNEL assay
Cells were seeded in six-well plates for 24 h and then treated with doxorubicin. After stimulation for 36 and 48 h, the cells in suspension and those attached to the plates were all harvested and resuspended in 600 μl PBS; 100 μl of the cell suspension were subjected to TUNEL staining by using an in situ cell death detection kit (Roche, Mannheim, Germany) in combination with 4’,6-diamidino-2-phenylindole (DAPI) staining. TUNEL-positive cells were counted in at least 300 cells in randomly chosen fields. The data were expressed as a percentage of TUNEL+ cells to total cells.
Cell counting kit-8 assay
To evaluate sensitivity of HCC cell lines to doxorubicin, the cells were seeded in 96-well plates at a density of 5 × 103 per well for 24 h, followed by treatment with doxorubicin at 18 different concentrations for 36 h. The concentration of doxorubicin ranged from 0.1 to 20 μ for SMMC-7721, and from 0.1 to 40 μ for MHCC97L. After 34 h of incubation, the cell counting kit reagent were added to the well and incubated for 2 h. Then the absorbance was recorded at 450 nm. The half-maximal inhibitory concentration (IC50) values were calculated by nonlinear regression analysis using the GraphPad Prism software (Version 6.0, GraphPad Software, Inc., San Diego, CA, USA).
Statistical analysis
All statistical analyses were performed by the Statistical Package for the Social Sciences (SPSS; Version 13.0, SPSS Institute, Chicago, IL, USA). Univariate and multivariate analyses were based on a Cox proportional hazard regression model. The χ2 test was used to analyze the distribution difference of Cbx4 expression among different clinicopathological features. The corresponding risk value OR and 95% CI were calculated using binary logistical regression with likelihood ratio test for forward method. Kaplan-Meier survival analysis (with log-rank test) was used to evaluate the relationship between Cbx4, VEGF or other clinicopathological factors and HCC prognosis. P-values <0.05 were considered statistically significant.
Abbreviations
- AFP:
-
α-fetoprotein
- CI:
-
confidence interval
- DFS:
-
disease-free survival
- DM:
-
distant metastasis
- HCC:
-
hepatocellular carcinoma
- HM:
-
hematogenous metastasis
- HR:
-
hazards ratio
- MVD:
-
microvessel density
- OR:
-
odds ratio
- OS:
-
overall survival
- TACE:
-
transarterial chemoembolization
- TAE:
-
transarterial embolization
- TNM:
-
tumor-node-metastasis
- VEGF:
-
vascular endothelial growth factor
References
Forner A, Llovet JM, Bruix J . Hepatocellular carcinoma. Lancet 2012; 379: 1245–1255.
Singal AG, Pillai A, Tiro J . Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624.
Vilarinho S, Calvisi DF . New advances in precision medicine for hepatocellular carcinoma recurrence prediction and treatment. Hepatology 2014; 60: 1812–1814.
Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP . Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer 2007; 43: 979–992.
Pang R, Tse E, Poon RT . Molecular pathways in hepatocellular carcinoma. Cancer Lett 2006; 240: 157–169.
Iakova P, Timchenko L, Timchenko NA . Intracellular signaling and hepatocellular carcinoma. Semin Cancer Biol 2011; 21: 28–34.
Feng GS . Conflicting roles of molecules in hepatocarcinogenesis: paradigm or paradox. Cancer Cell 2012; 21: 150–154.
Moeini A, Cornella H, Villanueva A . Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer 2012; 1: 83–93.
Yang L, Rong W, Xiao T, Zhang Y, Xu B, Liu Y et al. Secretory/releasing proteome-based identification of plasma biomarkers in HBV-associated hepatocellular carcinoma. Sci China Life Sci 2013; 56: 638–646.
Finn RS, Zhu AX . Targeting angiogenesis in hepatocellular carcinoma: focus on VEGF and bevacizumab. Expert Rev Anticancer Ther 2009; 9: 503–509.
Sampat KR, O'Neil B . Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist 2013; 18: 430–438.
Pazo-Cid RA, Lanzuela M, Esquerdo G, Perez-Gracia JL, Anton A, Amigo G et al. Novel antiangiogenic therapies against advanced hepatocellular carcinoma (HCC). Clin Transl Oncol 2012; 14: 564–574.
Lencioni R . Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010; 52: 762–773.
Forner A, Gilabert M, Bruix J, Raoul JL . Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014; 11: 525–535.
Farinati F, Vanin V, Giacomin A, Pozzan C, Cillo U, Vitale A et al. BCLC-B hepatocellular carcinoma and transcatheter arterial chemoembolization: a 20-year survey by the Italian Liver Cancer group. Liver Int 2014; 35: 1478–3223.
Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011; 53: 1580–1589.
Cucchetti A, Djulbegovic B, Tsalatsanis A, Vitale A, Hozo I, Piscaglia F et al. When to perform hepatic resection for intermediate stage hepatocellular carcinoma. Hepatology 2015; 61: 905–914.
Forner A, Llovet JM, Bruix J . Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol 2012; 56: 984–986.
Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2013; 260: 329–340.
Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One 2013; 8: e68193.
Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Pai JT et al. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol 2012; 19: 842–849.
Li F, Guo Z, Zhang Y, Wang H, Zhang X, Si T et al. Postoperative adjuvant arterial chemoembolization improves the survival of hepatitis B virus-related hepatocellular carcinoma: a retrospective control study. Ir J Med Sci 2014; e-pub ahead of print 28 June 2014; doi:10.1007/s11845-014-1164-6.
Liu H, Zhang A, Qian N, Gao L, Xu L, Zhang W et al. Postoperative transarterial chemoembolization benefits patients with high gamma-glutamyl transferase levels after curative hepatectomy for hepatocellular carcinoma: a survival stratification analysis. Tohoku J Exp Med 2012; 227: 269–280.
Zhong JH, Li LQ . Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: a meta-analysis. Hepatol Res 2010; 40: 943–953.
Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM, Sung CO et al. A genomic portrait of resectable hepatocellular carcinomas: Implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 2014; 60: 329–340.
Volkel P, Angrand PO . The control of histone lysine methylation in epigenetic regulation. Biochimie 2007; 89: 1–20.
Simon JA, Kingston RE . Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol 2009; 10: 697–708.
Schwartz YB, Pirrotta V . Polycomb complexes and epigenetic states. Curr Opin Cell Biol 2008; 20: 266–273.
Gil J, O'Loghlen A . PRC1 complex diversity: where is it taking us? Trends Cell Biol 2014; 24: 632–641.
Kagey MH, Melhuish TA, Wotton D . The polycomb protein Pc2 is a SUMO E3. Cell 2003; 113: 127–137.
Wotton D, Merrill JC . Pc2 and SUMOylation. Biochem Soc Trans 2007; 35: 1401–1404.
Nam SW, Park JY, Ramasamy A, Shevade S, Islam A, Long PM et al. Molecular changes from dysplastic nodule to hepatocellular carcinoma through gene expression profiling. Hepatology 2005; 42: 809–818.
Wang B, Tang J, Liao D, Wang G, Zhang M, Sang Y et al. Chromobox homolog 4 is correlated with prognosis and tumor cell growth in hepatocellular carcinoma. Ann Surg Oncol 2013; 20: S684–S692.
Li J, Xu Y, Long XD, Wang W, Jiao HK, Mei Z et al. Cbx4 governs HIF-1alpha to potentiate angiogenesis of hepatocellular carcinoma by its SUMO E3 ligase activity. Cancer Cell 2014; 25: 118–131.
Mei Z, Jiao HK, Wang W, Li J, Chen GQ, Xu Y . Polycomb chromobox 4 enhances migration and pulmonary metastasis of hepatocellular carcinoma cell line MHCC97L. Sci China Life Sci 2014; 57: 610–617.
Li J, Xu Y, Jiao HK, Wang W, Mei Z, Chen GQ . Sumoylation of hypoxia inducible factor-1alpha and its significance in cancer. Sci China Life Sci 2014; 57: 657–664.
Oliveri RS, Wetterslev J, Gluud C . Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev 2011; 16: CD004787.
Xi T, Yan ZL, Wang K, Li J, Xia Y, Shen F et al. Role of post-operative transcatheter arterial chemoembolization in hepatocellular carcinoma with different pathological characteristics. Zhonghua Wai Ke Za Zhi 2007; 45: 587–590.
Dong ZZ, Yao M, Wang L, Wu W, Gu X, Yao DF . Hypoxia-inducible factor-1alpha: molecular-targeted therapy for hepatocellular carcinoma. Mini Rev Med Chem 2013; 13: 1295–1304.
Zheng SS, Chen XH, Yin X, Zhang BH . Prognostic significance of HIF-1alpha expression in hepatocellular carcinoma: a meta-analysis. PLoS One 2013; 8: e65753.
Pelisch F, Pozzi B, Risso G, Munoz MJ, Srebrow A . DNA damage-induced heterogeneous nuclear ribonucleoprotein K sumoylation regulates p53 transcriptional activation. J Biol Chem 2012; 287: 30789–30799.
Friedrichs K, Gluba S, Eidtmann H, Jonat W . Overexpression of p53 and prognosis in breast cancer. Cancer 1993; 72: 3641–3647.
CSLC, CSCO. Diagnosis and treatment of primary liver cancer: a standardized expert consensus. Chin Clin Oncol 2009; 14: 259–269.
Acknowledgements
We appreciate Dr. J Wong at East China Normal University for providing plasmids. We also thank Y Ma, L Zeng, H Xu and M Wang for sample collection and management at the Department of Pathology, Affiliated Hospitals of Youjiang Medical College for Nationalities and Guangxi Medical University, for assisting with pathological techniques. This work was supported by grants from the National Natural Science Foundation of China (81430061 and 81472784), the National Basic Research Program of China (2015CB910403), Shanghai S&T Committee (11JC1406800) and Shanghai Committee of Education.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Oberst
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jiao, HK., Xu, Y., Li, J. et al. Prognostic significance of Cbx4 expression and its beneficial effect for transarterial chemoembolization in hepatocellular carcinoma. Cell Death Dis 6, e1689 (2015). https://doi.org/10.1038/cddis.2015.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2015.57
This article is cited by
-
Vessels that encapsulate tumor clusters (VETC) pattern predicts the efficacy of adjuvant TACE in hepatocellular carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
Mechanisms of Polycomb group protein function in cancer
Cell Research (2022)
-
Inhibiting CBX4 efficiently protects hepatocellular carcinoma cells against sorafenib resistance
British Journal of Cancer (2021)
-
RETRACTED ARTICLE: CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p
Molecular Cancer (2020)