Abstract
Exercise can be regarded as a drug for treating hypertension, and the ‘dosage’ (intensity/volume) is therefore of great importance. L-type voltage-gated Ca2+ (Cav1.2) channels on the plasma membrane of vascular smooth muscle cells have a pivotal role in modulating the vascular tone, and the upregulation of Cav1.2 channels is a hallmark feature of hypertension. The present study investigated the beneficial and adverse effects of exercise at different intensities on the remodeling of the Cav1.2 channel in mesenteric arteries (MAs) of spontaneously hypertensive rats (SHRs). Moderate- (SHR-M, 18–20 m min−1) and high-intensity (SHR-H, 26–28 m min−1) aerobic exercise training groups were created for SHRs and lasted for 8 weeks (1 h per day, 5 d per week). Age-matched sedentary SHRs and normotensive Wistar–Kyoto rats (WKY) were used as controls. The mesenteric arterial mechanical and functional properties were evaluated. Moderate-intensity exercise training induced a lower systolic blood pressure and heart rate in these rats compared with sedentary SHRs. BayK 8644 and nifedipine induced vasoconstriction and dose-dependent vasorelaxation, respectively, in the mesenteric arterial rings. Moderate-intensity exercise significantly suppressed the increase in BayK 8644-induced vasoconstriction, tissue sensitivity to nifedipine, Cav1.2 channel current density and Cav1.2 α1C-subunit protein expression in MAs from SHRs. However, high-intensity exercise training aggravated all of these hypertension-associated functional and molecular alterations of Cav1.2 channels. These results indicate that moderate-intensity aerobic training may act as a drug and effectively reverse the remodeling of Cav1.2 channels in hypertension to restore the vascular function in MAs, but that high-intensity exercise exaggerates the adverse remodeling of Cav1.2 channels and worsens the vascular function.
Similar content being viewed by others
Introduction
A growing body of evidence indicates that exercise is an effective nonpharmacological therapy for preventing and controlling cardiovascular diseases such as hypertension and arterial stiffness.1, 2, 3, 4 The beneficial effects of regular exercise (especially aerobic exercise) on general health and in reversing hypertension have been clearly shown.5 Therefore, it has been proposed that exercise be considered a drug. As with most drugs, the dosage is of great importance. An appropriate dosage should be chosen; otherwise, unfavorable side effects may occur. The health benefits of physical activity increase with an increasing amount of activity within a certain range; beyond a certain level, however, adverse effects will outweigh the benefits.6 Consequently, the ‘dosage’ (the volume and intensity of the exercise) must be taken into account in order to achieve the best clinical outcome. Although regular physical activity has a strong positive link to cardiovascular health, the dose response and maximum safe dose of physical activity, unlike those of chemical drugs, are still largely undefined.
Hypertension is a clinical syndrome characterized by increased arterial tone.7 In addition to the impaired endothelium-dependent vasodilatation, vascular smooth muscle cells (VSMCs) exhibit pathological alterations in response to persistent high intraluminal pressure. For instance, VSMCs are more depolarized as a consequence of the ion channel remodeling that occurs in chronic hypertension.8 L-type voltage-gated Ca2+ (Cav1.2) channels are the primary Ca2+ entry pathway in VSMCs; they regulate smooth muscle cell contractility and control regional organ blood flow and systemic blood pressure (BP).9, 10, 11, 12 Hypertension is associated with an elevation in arterial smooth muscle cell Cav1.2 channel protein and current density, leading to vasoconstriction.11, 12 Thus, pharmacological Cav1.2 channel blockers are an important therapeutic option for alleviating clinical hypertension. The administration of potent Cav1.2 channel blockers may efficiently attenuate or prevent the development of hypertension and its effects on target organs. Because exercise training is one of the most effective interventions prescribed for hypertensive individuals, it is extremely important to understand the effects of regular physiological exercise on the pathological alterations of Cav1.2 channels caused by arteriolar myocytes during hypertension. We hypothesized that appropriate intensity of exercise can lower BP and reverse the hypertension-associated Cav1.2 channel remodeling in peripheral resistant arterioles but that high-intensity exercise might exaggerate the adverse effects on Cav1.2 channel remodeling, thereby worsening vascular function and aggravating the hypertension.
Therefore, using a genetic hypertensive model of spontaneously hypertensive rats (SHRs), we investigated the effects of moderate- and high-intensity aerobic exercise training on Cav1.2 channel remodeling associated with hypertension. The information obtained will provide a better understanding of the relationship between exercise dosing and clinical outcomes, which is important for formulating public health recommendations.
Materials and methods
Animal protocol
Twelve-week-old male normotensive Wistar–Kyoto rats (WKY, n=18) and SHRs (n=54) were used. Animals in the hypertension group were randomly assigned into one of the following three groups: sedentary group (SHR, n=18), moderate-intensity exercise-trained group (SHR-M, n=18) and high-intensity exercise-trained group (SHR-H, n=18). All rats, including sedentary animals, were habituated to treadmill exercise. Each rat walked on a motor-driven treadmill at 15 m min−1 (0° slope) for 5 min per day for 5 days. The animals in the SHR-M and SHR-H groups ran on a motor treadmill (0° slope) at 18–20 m min−1 (~55–65% of the maximal aerobic velocity) and 26–28 m min−1 (~75–85% of the maximal aerobic velocity), respectively. Each exercise-trained group ran 60 min per day, 5 days per week for 8 weeks. Training volume (km per week) equals exercise intensity (m min−1) × duration (min per day) × frequency (days per week). Hence, because the training duration and frequency were identical among the training groups, the training volume was lower in the SHR-M group (5.4–6.0 km per week) than in the SHR-H group (7.8–8.4 km per week). To determine the maximal exercise capacity, the rats underwent a progressive exercise test using an incremental speed protocol of 5 m min−1 every 3 min and no grade until exhaustion. The rats were considered to be exhausted when they could no longer run at the treadmill speed.13 The treadmill exercise test was repeated after 4 weeks to adjust the training intensity.
All experimental protocols were approved by the ethical committee of the Beijing Sport University and were performed in accordance with the Chinese animal protection laws and institutional guidelines. Every effort was made to minimize the number of animals used and their suffering.
The body weight (BW) was measured weekly. The BP and heart rate (HR) were measured using an indirect tail–cuff method (BP-2010A, Softron Biotechnology, Beijing, China).
Isometric contraction studies
The mesenteric arteries (MAs) were removed after the rats were anesthetized with sodium pentobarbitone (60 mg kg−1, intraperitoneal) and were decapitated using a guillotine. The tissues were placed in cold Krebs’ solution with the following composition (mM): 131.5 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 13.5 NaHCO3 and 0.025 EDTA, and they were continuously gassed with 95% O2 and 5% CO2 (pH 7.4). Short segments of second-order branches (A2) were used for contractile studies with a Multi myograph system (620 M, DMT, Denmark).
The contractile response for tension was evaluated by measuring the maximum peak height and expressed as a percentage of contraction to 120 mM K+ (Kmax). To examine the effect of the Cav1.2 channel activator on resting tension, the vessel contractility was measured after the administration of 10−5 M BayK 8644 (a potent and effective calcium channel agonist). To examine the vasorelaxation effect of the Cav1.2 channel inhibitor, the tissues were first contracted with 10−5M norepinephrine (NE). Then, the responses to the cumulative addition of nifedipine (Cav1.2 channel blocker, 10−9–10−5M) were tested.
Electrophysiological experiments
Cell preparation
Cells were obtained with an enzymatic isolation method. The MAs were cut into small pieces and then incubated at 37 °C in 2 mg ml−g bovine serum albumin, 4 mg ml−1 papain, 1 mg ml−1 dithiothreitol and 0.6 mg ml−1 collagenase in physiological salt solution (in mM: NaCl 137, KCl 5.6, MgCl2 1, Glucose 10, HEPES 10, Na2HPO4 0.42, NaH2PO4 0.44, NaHCO3 4.2; pH 7.3; titrated with NaOH for 30–35 min). After enzymatic treatment, the vessel segments were washed with physiological salt solution and gently triturated to release individual smooth muscle cells with a fire-polished Pasteur pipette. Cells were cold-stored at 4 °C for up to 6 h until the experiments were performed.
Electrical recording
Whole-cell Cav1.2 currents were determined with a standard whole-cell voltage-clamp technique.14 Micropipettes (2–4 MΩ resistance with the recording solutions) were made from capillary glass (1.2 mm OD, 0.9 mm ID, WPI, Sarasota, FL, USA) with a programmable puller (PC-10, Narashige, Japan). Experiments were performed at 25 °C using 20 mM barium chloride as the charge carrier to limit the current rundown. The composition of the pipette solution was (in mM) as follows: CsCl 130, HEPES 10, Na2ATP 3, Na2GTP 0.1, MgCl2 1.5, Glucose 10, EGTA 10 and MgATP 0.5; pH 7.3; titrated with CsOH. The bath solution contained the following (in mM): BaCl2 20, HEPES 10, glucose 5, MgCl2 1 and choline chloride 124; pH 7.4; titrated with CsOH. The voltage protocol used to record IBa consisted of a holding potential of −80 mV and a sequence of pulses ranging from −70 to +70 mV, for a duration of 350 ms, in 10 mV increments. The cell capacitance was measured by applying a 5-mV test pulse and correcting transients with series resistance compensation. The series resistance was 70–80%, which was compensated to give a final value below 10 MΩ. Amplified currents were filtered at 2 kHz and acquired using a Digidata 1440 (Axon Instruments, Silicon Valley, CA, USA) analog to a digital converter acquisition board at 10 kHz. Data were analyzed using the PClamp10.2 software (Axon Instruments). In a subset of cells, 0.1 μM nifedipine was used to verify the identity of the Cav1.2 channel currents, and 5 μM BayK 8644 was used to activate the Cav1.2 channel currents. The effects of drugs were determined in the same cell by comparing currents before and after drug application.
To describe the voltage dependence of the activation of Cav1.2 channels, the current amplitude relative to its maximum value was fitted with a Boltzmann function that had the form P=1/{1+exp[(Vh—V)/k]}, where P is the current amplitude, measured as the ratio I/Imax and Imax is the maximum current amplitude of the whole-cell Cav1.2 currents. Vh is the potential for half-maximal activation, and k is a steepness factor (Boltzmann coefficient). To gain further insight into the time and voltage dependence of the inactivation of Cav1.2 channels, steady-state inactivation was determined using a standard two-step protocol. The protocol consisted of a 2000-ms conditioning prepulse to voltages from −100 to +50 mV, in steps of 10 mV, followed by a 40-ms test potential of +20 mV with a fixed 20-ms interpulse interval at the holding potential of −80 mV. Pulses were applied every 30 s. The steady-state inactivation was also measured as the ratio I/Imax, where Imax is the maximum current amplitude during the test pulse, after the most hyperpolarizing prepulse (−100 mV). The steady-state inactivation curve was drawn by fitting the data to a Boltzmann function with the form P=1/{1+exp[(V−Vh)/k]}, where P is the relative amplitude.
Immunofluorescence
For immunofluorescence staining, the VSMCs of MA were plated on poly-L-lysine-coated coverslips and fixed in 4% paraformaldehyde in phosphate-buffered saline for 20 min. Then, VSMCs were permeabilized with 0.2% Triton X-100 for 10 min. After extensive washing with phosphate-buffered saline, the VSMCs were blocked for nonspecific antibody binding with 5% bovine serum albumin (1 h), which was followed by overnight incubation at 4 °C in primary Rabbit polyclonal anti-α1C (Alomone Labs, Jerusalem, Israel) antibody, with 1% BSA, at a dilution of 1:200. The next day, after washing, a second Alexa Fluor 488 Goat Anti-Rabbit IgG antibody (Molecular Probes, Grand Island, NY, USA, 1:500) was used for 1 h in the dark. The VSMCs were also stained with 4′,6-diamidino-2-phenylindole at a dilution of 1:1000 for 10 min. Sample coverslips were plated on ProLong Gold Antifade Reagent (Molecular Probes) on the glass slides, and the edges were sealed. Images were acquired using a laser-scanning confocal microscope (TCS-SP5, Leica, Wetzlar, Germany) and analyzed using the ImageJ software (NIH, Bethesda, MD, USA, version1.46r).
Western immunoblotting
Membrane proteins were isolated and pooled for use in western blots as previously described.15 Briefly, equivalent amounts (20 μg) of total protein from the MAs were added to adjacent lanes. The antibody was polyclonal anti-α1C (1:200), which was purchased from Alomone Laboratories. After incubation with a secondary antibody (anti-rabbit IgG-HRP, 1:6000, Proteintech Group, Wuhan, Hubei, China) for 1 h, immunoreactive brands were visualized with enhanced chemiluminescence, and signals were recorded with Bio-Rad ChemiDOC XRS+ (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH was detected and used to correct for the equal loading of all samples. The protein band intensities were determined using the Quantity One (Bio-Rad) software. For quantification, the protein band intensities were first normalized to GAPDH and then to the appropriate control.
RT-PCR
MAs were placed in RNAlater (Ambion, Austin, TX, USA) at 4 °C. We isolated total RNA with a PureLink RNA Mini Kit (Ambion) and reverse-transcribed the RNA into cDNA with the GoScript Reverse Transcription System (Promega, Madison, WI, USA), according to the manufacturer’s instructions. Primers specific to L-type channel α1C (Cacna1c; GenBank accession no. NM_012517; amplicon=70 bp; forward: 5′-GAGAGCTTTCCGTGTGCTTC-3′ and reverse: 5′-GTTCAGGACCACCTGGAGAC-3′) and the housekeeping genes GAPDH (GenBank accession no. NM_017008; amplicon=104 bp; forward: 5′-CCAGGTTGTCTCCTGTGACTTC-3′ and reverse: 5′-ACCAGGAAATGAGCTTCACAAA-3′) were designed to identify the presence of each transcript in the mesenteric arterial vascular smooth muscle. PCR was performed in a RT-PCR machine (Bio-Rad) with Taq 2X Master Mix (New England Biolabs, Beverly, MA, USA). After initial denaturation for 3 min at 94 °C, PCR cycling included 32 cycles as follows: 94 °C for 30 s, 55 °C for 30 s and 72 °C for 60 s, which was followed by a final extension step at 72 °C for 5 min. Amplicons were verified using 1.5% agarose gel electrophoresis.
Chemicals
All chemicals were purchased from Sigma-Aldrich (China (Mainland)) unless otherwise stated.
Statistical analysis
Data were expressed as the mean±s.e.m. The term n represented the number of cells or animals used in each experiment. Where appropriate, differences were evaluated for statistical significance (P<0.05) by a one-way analysis of variance or t-test.
Results
Physical characteristics of the rats
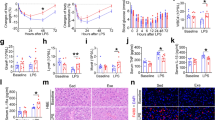
Among these animals, one rat in the SHR-H group died after exercise training in the second training week and could not be used for further analysis. There were no significant differences in the BWs between the WKY and SHR groups at the beginning of the study. However, at the end of the study, the BWs in the SHR exercise groups were significantly lower than those of their sedentary counterparts (Table 1), and the BW in the high-intensity group was markedly lower than that of the moderate-intensity group. Although the heart weight (HW) in the SHRs was higher than in WKY rats, no significant differences were observed among the hypertension groups. Similarly, the HW to BW ratio (HW/BW) in the SHRs was higher than that in the normotensive WKY rats, whereas in the SHR-H group (4.38±0.07), the HW/BW was higher than those of either the SHR-M group (4.19±0.10) or sedentary SHR group (4.03±0.07).
As shown in Table 1, the systolic blood pressure (SBP) and HR in the SHRs were significantly higher than those in the WKY rats. After 8 weeks of exercise, the SBP and HR in the SHR-M group were significantly lower than those in the sedentary SHR group. However, in the SHR-H group, the SBP was higher than that in SHRs. There was no significant difference in the HR between SHR-H and SHR groups. While compared with SHR-M group, the SBP and HR in the SHR-H group were markedly higher.
Moderate-intensity exercise abrogates but high-intensity exercise aggravates the role of Cav1.2 channel in regulating vascular tone in MAs for SHRs
To determine the Cav1.2 channel contribution to the vascular tone, the effects of the Cav1.2 channel activator or inhibitor on the contraction/relaxation of MA rings were measured. In each experiment, the nonselective nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM) was added 20 min before the contraction studies. The maximal response induced by KCl (120 mM) in L-NAME-treated animals was similar in the four groups. As shown in Figures 1Aa and B, BayK 8644 (10−5 M) elicited a tension increase in all groups that was much higher in SHRs than in WKY rats, whereas moderate-intensity exercise training (MIT, that is, ~55–65% of the maximal aerobic velocity) markedly inhibited this increase. Interestingly, high-intensity exercise training (HIT, that is, ~75–85% of the maximal aerobic velocity) significantly augmented the BayK 8644-induced tension increase.
Effects of the Cav1.2 channel activator or inhibitor on the vascular tension in MAs from the WKY, SHR, SHR-M and SHR-H groups. (A) Typical experimental tracings showing the following: (a) vascular tone induced by BayK 8644 (10−5 M) and (b) effect of the Cav1.2 channel inhibitor nifedipine (10−9–10−5 M) on NE-induced vessel contraction. In each experiment, the arteries were preincubated with the nonselective nitric oxide synthase inhibitor L-NAME (100 μM) for 20 min (dotted arrows). (B) Statistic diagram of BayK 8644-induced vessel contraction. (C) Concentration–response curves of nifedipine on NE (10−5 M)-induced contraction. *P<0.05, vs. WKY; #P<0.05, vs. SHR; and &P<0.05, vs. SHR-M. n=6 in each group. BayK, BayK 8644 and Nif, nifedipine.
NE (10−5M) also induced a significant increase in vascular tone. The maximal NE-induced force in SHRs was 132.2±6.7%Kmax, which was higher than that of the WKY (105.6±5.8%Kmax, P<0.01) and SHR-M (114.3±5.2%Kmax, P<0.01) groups. However, after HIT, the NE-induced maximal tension increase (SHR-H, 142.4±6.5%Kmax) was significantly higher than that of SHR-M (Figures 1Ab and B). As shown in Figure 1Ab, at the plateau of NE-induced contraction, nifedipine (10−9–10−5 M) was administered in half-log increments. A parallel leftward shift of the concentration–relaxation curve was detected in hypertensive rats. Here, the 10−5-M NE-induced maximal tension increase was treated as 100% in each group. The pIC50 values (negative logarithm of the molar concentration required to block 50% of the NE-induced contraction) in the four groups were 7.14±0.14 (WKY), 7.56±0.07 (SHR), 7.35±0.09 (SHR-M) and 7.80±0.07 (SHR-H; n=6 each group, Figure 1C). Therefore, the ranking of the sensitivity of the MA to nifedipine was SHR-H>SHR>SHR-M>WKY (all P<0.05). These results indicated that the contribution of the Cav1.2 channels in vascular tone regulation was significantly increased in SHRs but that MIT significantly ameliorated while HIT significantly augmented this change.
Moderate-intensity exercise inhibits, but high-intensity exercise enhances, the SHR-associated increase in Cav1.2 channel currents in MAs
The electrophysiological properties of Cav1.2 channels in myocytes from small MAs were determined. Figure 2A illustrates the typical records of the whole-cell Ca2+ currents recorded in VSMCs isolated from MAs. The peak inward current was recorded at +20 mV in all four groups. BayK 8644 (5 μM) augmented the peak inward Ba2+ current (IBa) and negatively shifted the I–V relationship (peak Cav1.2 current densities shifted to 0 mV), whereas nifedipine (0.1 μM) almost completely suppressed the inward currents. These properties suggest that the recorded inward currents were Ba2+ currents through Cav1.2 channels. To obtain the I–V curve of Cav1.2, the current densities were plotted against the corresponding command potentials (Figure 2B). The membrane Ba2+ currents are expressed relative to the cell capacitance to compensate for differences in the cell size (pA/pF). The mean cell capacitance for WKY (17.2±1.3 pF, n=20 cells per six rats) and SHR (17.5±1.5 pF, n=22 cells per six rats) cells were similar and were not significantly changed by exercise training (SHR-M, 16.8±1.6 pF, n=24 cells per six rats; SHR-H, 17.1±1.6 pF, n=18 cells per six rats; P>0.05 when comparing all). The mean peak Cav1.2 current density was −18.5±2.1 pA/pF in hypertensive SHR cells compared with −9.1±0.3 pA/pF in WKY cells; the former was thus approximately twofold larger (Figure 2 and Table 2). However, the Cav1.2 current densities were significantly different for different exercise-training intensities. MIT reduced the peak Cav1.2 current density in SHR cells to −11.6±1.1 pA/pF (or by ~62.7%), and HIT increased it to −27.6±2.7 pA/pF (or by ~149.2%).
Whole-cell Cav1.2 currents recorded in the myocytes of the MAs from the WKY, SHR, SHR-M and SHR-H groups. Arterial myocytes were freshly isolated from the MAs. (A) Traces of Ca2+ channel currents evoked by command potentials (−70 to +70 mV in a 10 mV step) in the absence (a) or presence of BayK 8644 (b, 5 μM) or nifedipine (c, 0.1 μM). (B) Current–voltage relationships of the Cav1.2 currents in the VSMCs. The current amplitudes at various command potentials were normalized by the cell capacitance and plotted. (C) Steady-state inactivation and activation curves of the Ba2+ currents in mesenteric myocytes from the WKY, SHR, SHR-M and SHR-H myocytes.
The activation and steady-state inactivation curves of mesenteric Cav1.2 channels and the mean values of the Boltzmann fit parameters are summarized in Figure 2C and Table 2. The voltage dependence of the half-maximal Cav1.2 current activation (Vh) and slope (k) were similar in the WKY, SHR, SHR-M and SHR-H myocytes. The voltage dependence of the half-maximal inactivation and slope were also similar in the four groups. All of these data indicated that hypertension was associated with an enhancement of the functional expression of Cav1.2 channels. MIT attenuated these changes, whereas HIT exaggerated the adverse functional remodeling of Cav1.2 channels in MA myocytes from SHRs.
Moderate-intensity exercise suppresses, but high-intensity exercise augments, the upregulation of Cav1.2 channel α1C protein expression in the MAs from SHRs
To investigate the molecular mechanism underlying the Cav1.2 current modifications, we examined the Cav1.2 channel α1C subunit protein expression by immunohistochemical staining and western blotting.
Mesenteric myocytes were freshly isolated for immunocytochemical analysis, and the fluorescence intensities of a total of 32 cells were analyzed individually. As shown in Figures 3A and B, the fluorescence signals from the SHR cells labeled with the anti-α1C antibody were higher than those in the WKY cells when exposed for equivalent times, showing a significantly increased average intensity of ~2.9-fold in the SHRs. Similarly, MIT suppressed this alteration (~1.5-fold of WKY), but HIT reinforced it (~3.1-fold of WKY).
Fluorescent microphotographs of confocal microscopy images and quantitative analysis of Cav1.2 α1C subunit arterial myocytes. (Aa) Representative positive immunostaining of the α1C subunit in isolated VSMCs from the WKY, SHR, SHR-M and SHR-H groups. (b) Blue indicates 4′,6-diamidino-2-phenylindole (DAPI) staining of the nuclei. (c) Overlay images with the α1C subunit (green) and DAPI (blue). (d) Bright field image of the VSMCs after staining. (e) Representative image of VSMCs stained with α1C antibody+blocking peptide. (B) The mean data illustrating the fluorescence intensity of the α1C subunit (WKY). *P<0.05 vs. WKY; #P<0.05 vs. SHR; and &P<0.05 vs. SHR-M.
Subsequently, western blot analysis was used to compare the expression of the α1C subunit protein in the four groups (Figures 4a and b). The α1C subunit was overexpressed in the mesenteric myocytes of SHRs (3.51±0.25, n=6) compared with WKY rats (1.00±0.00, n=6), which was ameliorated by MIT (SHR-M, 2.51±0.31, n=6). However, HIT significantly increased, rather than inhibited, the α1C protein expression (6.51±0.50, n=5) compared with its sedentary counterparts (all P<0.05).
Protein expression of the Cav1.2 channel (α1C−) subunit in the mesenteric smooth muscle cells. (a) Immunoreactive bands corresponding to the α1C− subunit and GAPDH detected by western blot analysis (left panel). Right panel, the α1C− subunit was recognized in the left lane as a 200-kDa band in the MAs of WKY rats (−CP), but it was absent in the right lane after competing peptide inhibition (+CP). (b) Summarized data of the α1C− subunit protein levels expressed as a ratio to the GAPDH levels (n=6 for the WKY, SHR and SHR-M groups; n=5 for SHR-H). (c) Representative agarose gel showing the Cav1.2 channel α1C− subunit and GAPDH (control) mRNA amplicons from the arterial myocytes in the four groups. (d) The mean PCR data for the Cav1.2 channel α1C mRNA in the MAs normalized to GAPDH and then to age-matched WKY controls. n=6 in each group. *P<0.01 compared with WKY rats; #P<0.01 compared with SHRs; and &P<0.05 vs. SHR-M.
Moderate-intensity exercise reverses, but high-intensity exercise aggravates, the upregulation of the Cav1.2 channel α1C mRNA in the MAs from SHRs
We also examined the Cav1.2 channel α1C expression at the transcriptional level using conventional RT-PCR (Figures 4c and d). GAPDH was used as the reference gene. The results showed that the mean α1C mRNA levels were ~2.5-fold (2.53±0.18, n=6) higher in SHRs compared with the arteries from WKY rats (1.00±0.00, n=6). However, MIT significantly reversed (1.08±0.13, n=6), but HIT markedly aggravated (3.14±0.17, n=6) this elevation.
Discussion
In our recent study, we demonstrated that chronic aerobic exercise can normalize the changes in the Cav1.2 and Kca1.1 channels in the MAs from SHRs.16 The present study focuses primarily on the effect of the exercise intensity/volume on Cav1.2 channel remodeling in arterioles from SHRs. Consistent with our hypothesis, this study provides the initial functional and molecular evidence that high-intensity exercise aggravates hypertension and exaggerates the adverse remodeling of Cav1.2 channels in mesenteric arterial smooth muscle cells from SHRs, whereas moderate-intensity exercise reduces the BP and reverses the Cav1.2 channel remodeling on arteriole myocytes.
SHRs exhibited a higher HR, pressure and pulse pressure compared with WKY rats. Although it was recently proposed that exercise can be considered a drug, it is not easy to issue a public recommendation for physical activity in patients with hypertension.2 The dosage (volume and intensity of the exercise), frequency of administration (sessions per week), type (aerobic vs. resistance exercise) and side effects of exercise must be taken into account to achieve the best clinical outcome.
In the present study, at the end of the 8 weeks of moderate-intensity aerobic exercise training, rats in the SHR-M group had a decreased SBP and HR, suggesting that an appropriate intensity of exercise training may be an effective therapy for hypertension. This decrease in the BP may be attributed to the attenuation of the sympathetic activity to the heart, which can lead to bradycardia and, consequently, a reduction in the cardiac output.17, 18 This lower sympathetic activity induced by exercise training could also be attributed to an improvement of the arterial baroreflex and chemosensitive cardiopulmonary baroreflex sensitivity in SHRs,18, 19, 20, 21 which modulates the peripheral autonomic nervous system. In addition, other peripheral mechanisms are very important. The small arteries and arterioles undergo extensive biological and structural adaptations in response to the elevated intraluminal perfusion pressure that occurs during chronic hypertension. The underlying pathophysiological processes appear to be complex, and they likely involve vascular remodeling, endothelial dysfunction, smooth muscle cell hypertrophy and changes in the extracellular matrix composition and function.22, 23 The net effect of these adaptive changes is augmented vasoconstriction and impaired vascular relaxation to various physiological stimuli, which result in elevated vascular tone in the arteries and arterioles.24, 25 Azevedo et al.26 demonstrated that the observed BP decrease in moderate-intensity treadmill-trained SHRs was due to the attenuation in the total peripheral vascular resistance.26 The probable mechanisms of this reduction include an improvement in endothelial function that occurs after increasing the shear stress and stimulating an effect on the production of nitric oxide by the endothelium.27 In addition, the pathological vascular remodeling in the arteries and arterioles during hypertension is markedly suppressed.
The benefits of exercise on cardiovascular health are almost certain; however, the negative effects of exercise overdose on cardiovascular function are largely unknown. One previous study showed that high-intensity aerobic exercise may accelerate hypertensive heart disease and improve fibrosis.28 In our study, one animal in the SHR-H group died after one session of high intensity of exercise training. Moreover, the BP and HR in the SHR-H group did not decrease; instead, they increased significantly compared with the sedentary SHR group. These results are in line with the results obtained by da Costa Rebelo et al.,28 who suggested that high-intensity aerobic exercise must be considered an important risk factor rather than a therapeutic intervention.
There are considerable data in the literature suggesting that augmented Ca2+ influx through the Cav1.2 channels contributes to the augmented peripheral resistance and contractile responses of the vascular smooth muscle in hypertension. Using vascular isometric contraction recording along with Cav1.2 channel-specific pharmacological activators (BayK 8644) and blockers (nifedipine), we demonstrated the enhanced function of the Cav1.2 channels in the arterioles of SHRs, which was in line with previous reports.29, 30 However, at the end of MIT, both the NE-induced vasoconstriction and the sensitivity of the MA to nifedipine were decreased compared with untreated SHRs, thus providing strong support that appropriate exercise may correct the upregulation of the Cav1.2 channel function in maintaining vascular tone in SHRs. Interestingly, HIT induced the opposite effects. NE-induced vasoconstriction increased even more in the SHR-H group than in sedentary SHRs, which indicated that the hypertension-associated increase in the vasoconstriction in response to vasoconstrictors became even heavier after HIT. Both BayK 8644-induced vasoconstriction and the sensitivity of MA to nifedipine were higher in the SHR-H group than in SHRs, indicating that the role of the Cav1.2 channel in vascular tension regulation was elevated even more for an overdose of exercise training.
Previous electrophysiological studies have demonstrated elevated Ca2+ currents in freshly isolated VSMCs from cerebral, mesenteric, renal, skeletal and pulmonary arteries in various hypertensive animal models.29, 30, 31 In addition, the enhanced amplitude of the whole-cell current is attributable to the increased number of the Cav1.2 channel openings instead of the change in the single channel conductance, open-time distribution or voltage sensitivity.32 In the present study, we found that the peak Cav1.2 currents were about twice as high in the mesenteric arterial myocytes of SHRs than in those of WKY rats, which was in agreement with previous reports.29, 30, 31, 33, 34 Some previous studies have also investigated the effects of exercise on the Cav1.2 channels in specific arteries from normal animals. Bowles et al.35 demonstrated that the smooth muscle L-type Ca2+ current density is increased within the coronary arterial bed by endurance exercise training in miniature swines. This increased voltage-gated calcium channel density may provide an important mechanistic link between functional and cellular adaptations in the coronary circulation in response to exercise training. However, in hypertensive animals, the Ca2+ currents in myocytes from various arteries are significantly increased.29, 30, 31 Will exercise training continue to increase the Ca2+ currents or suppress it instead? No answer can be found in previous reports. In the present study, we demonstrated that after a period of MIT, this increase in the Cav1.2 channel functional expression was effectively ameliorated. However, after HIT, the discrepancy in the Cav1.2 current density between WKY rats and SHRs increased, thus providing further evidence that HIT accelerates the adverse remodeling of the Cav1.2 channels in MA myocytes from hypertension. No changes were observed in the voltage dependence activation and inactivation kinetics among the four groups.
Immunostaining in single myocytes and the western blot assay showed that the molecular expression of the pore-forming α1C subunit of the Cav1.2 channel was significantly elevated in the arteries of hypertensive animals compared with age-matched normotensive animals. This finding indicates that the profound upregulation in the vascular Cav1.2 channel function observed in hypertensive animals is largely because of the increased channel expression. With respect to the relationship between the exercise dose and effects, we found, for the first time, that MIT significantly inhibited the protein expression of the α1C subunit protein, which was associated with hypertension. Conversely, HIT exaggerated this pathological remodeling of the Cav1.2 channels in MA myocytes. All of these immunostaining and western blotting results strongly supported the functional study.
At the mRNA level, we also found that the Cav1.2 α1C mRNA levels in the MAs were higher in SHRs than in WKY rats. Obviously, the increase in α1C (~2.5-fold) mRNA cannot fully account for the elevation in its protein (~3.5-fold) during hypertension. These data indicate that both transcriptional and post-translational mechanisms elevate α1C proteins in the MA myocytes during hypertension. In other words, in addition to transcriptional activity, post-transcriptional mechanisms, such as increased translation efficiency, increased trafficking of channel proteins to the plasma membrane and increased stability of the channel protein complex, may contribute to the upregulation of α1C protein in the vasculature during hypertension.36 After MIT, α1C decreased to ~1.1-fold of WKY rats at the mRNA level; however, at the protein level, α1C decreased to ~2.5-fold of WKY rats. By contrast, after HIT, α1C increased to ~3.1-fold of WKY rats at the mRNA level, whereas it increased to ~6.5-fold of WKY rats. These results suggest that exercise training affects the post-transcriptional processes much more than the transcriptional activity of Cav1.2 α1C in the MAs from SHRs.
Data presented in the literature show that there is a strong positive correlation between the BP and number of functional Cav1.2 channels in the VSMCs in vivo.27 For instance, the SBP was found to be linearly correlated to the membrane densities of the Cav1.2 channel currents in VSMCs from the small MAs of SHRs and WKY rats.31 This may be an explanation for the results of the present study, that is, a reduction in the SBP by treatment with moderate-intensity aerobic exercise training resulted in a concomitant decrease in the Cav1.2 channel current densities in the VSMCs of SHRs. In contrast, an aggravated high BP resulted in a concomitant exaggerated increase in the Cav1.2 channel current densities. However, how is this process initiated? What comes first—BP-lowering or a reduction in the number of calcium channels? The answer to this question is not clear. The results of these experiments alone cannot answer this question, and more studies are needed to clarify it. However, in the present study, we provide solid evidence that exercise training may regulate the Cav1.2 channel function and pore-forming α1C subunit molecular expression, which is closely related to the exercise-training intensity.
There are several notable limitations in the present study. First, we only examined the pore-forming α1C subunit molecular expression at the protein and mRNA levels. Cav1.2 channels consist of pore-forming α1 and auxiliary α2δ and β subunits.37 It is still unclear whether these auxiliary subunits are changed during hypertension and whether MIT and HIT have different effects on their subunit expression. Recently, it has been reported that genetic hypertension is associated with an elevation in the α2δ-1 expression, which promotes the surface trafficking of the Cav1.2 channels in cerebral artery myocytes.11 Recently, α2δ-1 was identified as critical for the functional trafficking of Cav1.2 α1 subunits to the plasma membrane (surface) in arterial myocytes.38 In addition, it has been reported that the β3 subunit is a critical regulatory protein that is required to upregulate arterial Cav1.2 channels and fully develop Ang II-dependent hypertension in C57BL/6 mice.39 Obviously, post-translational alterations of the α1C subunit by regulatory β and α2σ subunits may also alter the expression of Cav1.2 by regulating its formation or its targeting and stability in the plasma membrane.40, 41 At the moment, it is unclear to what extent the α2σ and β subunits are expressed and functionally important for the changes in the function of VSMCs as well as which factors affect their interaction with the α1C subunit. Therefore, further studies will be needed to determine the exact molecular basis of this abnormality. Another limitation that should be mentioned is that in the present study we did not investigate the effects of the exercise intensity on the Cav1.2 function in normotensive (WKY) animals. According to the present data, it is difficult to interpret the cause–effect relationship between changes in the calcium channel function/expression and exercise intensity with concurrent changes in the BP. To answer this question, future studies should be conducted by repeating the same protocol in normotensive control animals.
Conclusion
In summary, using a combination of mechanical, electrophysiological and biochemical approaches, the present study provided functional and molecular evidence that moderate-intensity aerobic exercise training reverses pathological Cav1.2 channel remodeling, inducing vessel dilation in hypertension, whereas high-intensity aerobic exercise training aggravates the adverse remodeling of Cav1.2 channels in association with hypertension. These data indicate that for the intervention treatment of hypertension an appropriate training protocol should be chosen. The study may contribute to optimize the treatment regimens of hypertensive patients who are performing aerobic exercise. Further studies on the regulation of Cav1.2 channel transcription, translation and trafficking should provide more insights into the molecular mechanisms.
References
Hagberg JM, Park JJ, Brown MD . The role of exercise training in the treatment of hypertension. Sports Med 2000; 30: 193–206.
Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC . Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol 2012; 167: 1–12.
Heffernan KS, Yoon ES, Sharman JE, Davies JE, Shih YT, Chen CH, Fernhall B, Jae SY . Resistance exercise training reduces arterial reservoir pressure in older adults with prehypertension and hypertension. Hypertens Res 2013; 36: 422–427.
Doonan RJ, Mutter A, Egiziano G, Gomez YH, Daskalopoulou SS . Differences in arterial stiffness at rest and after acute exercise between young men and women. Hypertens Res 2013; 36: 226–231.
Vina J, Borras C, Sanchis-Gomar F, Martinez-Bello VE, Olaso-Gonzalez G, Gambini J, Ingles M, Gomez-Cabrera MC . Pharmacological properties of physical exercise in the elderly. Curr Pharm Des 2014; 20: 3019–3029.
Lee IM . Dose-response relation between physical activity and fitness: even a little is good; more is better. JAMA 2007; 297: 2137–2139.
Beevers G, Lip GYH, O’Brien E . The pathophysiology of hypertension. BMJ 2001; 322: 912–916.
Harder DR, Brann L, Halpern W . Altered membrane electrical properties of smooth muscle cells from small cerebral arteries of hypertensive rats. Blood Vessels 1983; 20: 154–160.
Amberg GC, Rossow CF, Navedo MF, Santana LF . NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem 2004; 279: 47326–47334.
Cartin L, Lounsbury KM, Nelson MT . Coupling of Ca2+ to CREB activation and gene expression in intact cerebral arteries from mouse: roles of ryanodine receptors and voltage-dependent Ca2+ channels. Circ Res 2000; 86: 760–767.
Gollasch M, Nelson MT . Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res 1997; 20: 355–371.
Jaggar JH, Porter VA, Lederer WJ, Nelson MT . Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 2000; 278: C235–C256.
Roque FR, Briones AM, García-Redondo AB, Galán M, Martínez-Revelles S, Avendaño MS, Cacholfeiro V, Fernances T, Vassallo DV, Oliveira EM, Salaices M . Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol 2013; 168: 686–703.
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ . Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 1981; 391: 85–100.
Shi L, Liu X, Li N, Liu B, Liu Y . Aging decreases the contribution of MaxiK channel in regulating vascular tone in mesenteric artery by unparallel downregulation of α- and β1-subunit expression. Mech Ageing Dev 2013; 134: 416–425.
Shi L, Zhang H, Chen Y, Liu Y, Lu N, Zhao T, Zhang L . Chronic exercise normalizes changes of Cav1.2 and KCa1.1 channels in mesenteric arteries from spontaneously hypertensive rats. Br J Pharmacol 2014; 172: 1846–1858.
Negrão CE, Irigoyen MC, Moreira ED, Brum PC, Freire PM, Krieger EM . Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Am J Physiol 1993; 265: R365–R370.
Véras-Silva AS, Mattos KC, Gava NS, Brum PC, Negrão CE, Krieger EM . Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats. Am J Physiol 1997; 273: H2627–H2631.
Krieger EM, Brum PC, Negrão CE . State-of-the-Art lecture: influence of exercise training on neurogenic control of blood pressure in spontaneously hypertensive rats. Hypertension 1999; 34: 720–723.
Silva GJ, Brum PC, Negrão CE, Krieger EM . Acute and chronic effects of exercise on baroreflexes in spontaneously hypertensive rats. Hypertension 1997; 30: 714–719.
Zamo FS, Barauna VG, Chiavegatto S, Irigoyen MC, Oliveira EM . The renin–angiotensin system is modulated by swimming training depending on the age of spontaneously hypertensive rats. Life Sci 2011; 89: 93–99.
Mehta PK, Griendling KK . Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007; 292: C82–C97.
Touyz RM . Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol 2005; 90: 449–455.
Baumbach GL, Heistad DD . Remodeling of cerebral arterioles in chronic hypertension. Hypertension 1989; 13: 968–972.
Heistad DD, Mayhan WG, Coyle P, Baumbach GL . Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels 1990; 27: 258–262.
Azevedo LF, Brum PC, Mattos KC, Junqueira CM, Rondon MU, Barretto AC, Negrão CE . Effects of losartan combined with exercise training in spontaneously hypertensive rats. Braz J Med Biol Res 2003; 36: 1595–1603.
Sherman DL . Exercise and endothelial function. Coron Artery Dis 2000; 11: 117–122.
da Costa Rebelo RM, Schreckenberg R, Schlüter KD . Adverse cardiac remodelling in spontaneously hypertensive rats: acceleration by high aerobic exercise intensity. J Physiol 2012; 590: 5389–5400.
Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ . Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol 2006; 44: 131–142.
Pesic A, Madden JA, Pesic M, Rusch NJ . High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal. Circ Res 2004; 94: e97–e104.
Lozinskaya IM, Cox RH . Effects of age on Ca2+ currents in small mesenteric artery myocytes from Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 1997; 29: 1329–1336.
Ohya Y, Abe I, Fujii K, Takata Y, Fujishima M . Voltage dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ Res 1993; 73: 1090–1099.
Asano M, Masuzawa-Ito K, Matsuda T . Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br J Pharmacol 1993; 108: 214–222.
Rusch NJ, Runnells AM . Remission of high blood pressure reverses arterial potassium channel alterations. Hypertension 1994; 23: 941–945.
Bowles DK, Hu Q, Laughlin MH, Sturek M . Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 1998; 275: H2159–H2169.
Joseph BK, Thakali KM, Moore CL, Rhee SW . Ion channel remodeling in vascular smooth muscle during hypertension: implications for novel therapeutic approaches. Pharmacol Res 2013; 70: 126–138.
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J . International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 2005; 57: 411–425.
Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH . Smooth muscle cell α2δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res 2009; 105: 948–955.
Kharade SV, Sonkusare SK, Srivastava AK, Thakali KM, Fletcher TW, Rhee SW, Rusch NJ . The β3 subunit contributes to vascular calcium channel upregulation and hypertension in angiotensin II-infused C57BL/6 mice. Hypertension 2013; 61: 137–142.
Gao T, Chien AJ, Hosey MM . Complexes of the alpha1C and beta subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J Biol Chem 1999; 274: 2137–2144.
Schleithoff L, Mehrke G, Reutlinger B, Lehmann-Horn F . Genomic structure and functional expression of a human α2/σ calcium channel subunit gene (CACNA2). Genomics 1999; 61: 201–209.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31371201), the Beijing Natural Science Foundation (5132017), the Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-11-0850); the 2015 Chinese Universities Scientific Fund, the ‘12th Five-Year Plan’ (2012BAK21B03), and the 2013 Creative Research Group Program of Beijing Sport University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, H., Zhang, Y. et al. Exercise intensity-dependent reverse and adverse remodeling of voltage-gated Ca2+ channels in mesenteric arteries from spontaneously hypertensive rats. Hypertens Res 38, 656–665 (2015). https://doi.org/10.1038/hr.2015.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.56
Keywords
This article is cited by
-
Progressive aortic stiffness in aging C57Bl/6 mice displays altered contractile behaviour and extracellular matrix changes
Communications Biology (2022)
-
Increased Ca2+ content of the sarcoplasmic reticulum provides arrhythmogenic trigger source in swimming-induced rat athlete’s heart model
Scientific Reports (2020)
-
Exercise and Ion-Channel Remodeling in Vascular Smooth Muscle During Hypertension: Therapeutic Implications
Journal of Science in Sport and Exercise (2019)
-
The effects of anti-hypertensive drugs and the mechanism of hypertension in vascular smooth muscle cell-specific ATP2B1 knockout mice
Hypertension Research (2018)
-
BKCa channel activity and vascular contractility alterations with hypertension and aging via β1 subunit promoter methylation in mesenteric arteries
Hypertension Research (2018)