Abstract
Objective:
To determine the efficacy of heparinized saline administered as intermittent flush on functional duration of the peripheral intravenous catheter (PIVC) in neonates.
Study Design:
Randomized, double-blind and placebo-controlled trial. Setting: Neonatal intensive care unit of a teaching hospital. Participants: Term and preterm neonates born at >32 weeks of gestation who required PIVC only for intermittent administration of antibiotics. Intervention: Eligible neonates were randomized to receive 1 ml of either heparinized saline (10 U ml−1) (n=60) or normal saline (n=60) every 12 h before and after intravenous antibiotics. Main outcome measure: Functional duration of first peripheral intravenous catheter.
Result:
A total of 120 neonates were randomized to two groups of 60 neonates each. The mean (s.d.) of age of babies in case and control group was 5.7 (2.5) days and 4.6 (3.1) days, respectively. The average weight of babies in both the groups was 2.1 kg. Mean functional duration of first catheter was more in heparinized saline group, mean (s.d.) of 71.68 h (27.3) as compared with 57.7 h (23.6) in normal saline group (P<0.005). The mean (95% confidence interval) difference in functional duration in the two groups was 13.9 h (4.7–23.15). Mean duration of patency for any catheter was also significantly more in heparinized saline group than control group.
Conclusion:
Heparinized saline flush increases the functional duration of peripheral intravenous catheter.
Similar content being viewed by others
Introduction
Neonates with systemic sepsis, meningitis or septic-arthritis often need long-term intravenous (IV) antibiotic therapy. Peripheral IV catheter (PIVC) in such neonates have intermittent usage and tend to get blocked by blood clot at its distal tip, prompting frequent reinsertions. Repeated skin breach exposes the vulnerable newborns to infection and painful experiences which might potentially affect neurodevelopmental outcome.1 Although limb immobilization with splint2 at cannula site has not been shown to prolong the patency of PIVC, Heparin, acting as an anticoagulant, has been hypothesized to prolong the functional duration of a PIVC and reduce the number of catheterization attempts. There have been conflicting results of benefits of heparin lock in neonates.3 Cochrane meta-analysis reported inconclusive evidence for use of heparinized saline lock in prolonging duration of catheter in neonates and recommended that more studies are needed.3 We therefore conducted this study with the objective to evaluate the efficacy and safety of heparinized saline flush in concentration of 10 U ml−1 on functional duration of PIVC among term and preterm neonates.
Materials and methods
This randomized controlled trial was conducted in neonatal unit of a teaching hospital of North India from October 2009 to September 2010. Study protocol was cleared by Institutional Ethical Committee of the hospital. Informed written consent was obtained from either parents before enrollment. Term and preterm neonates born at >32 weeks of gestation who required peripheral IV catheter for intermittent administration of antibiotics with expected duration of hospital stay >5 days were eligible for enrollment in the study. Neonates with intracranial hemorrhage, platelet count <150 000 mm−3,4 coagulopathy or major congenital malformation were excluded.
Allocation of group was done by block randomization. Randomization sequence was generated in blocks of 8 using a computer program. Allocation sequences were then placed in serially numbered, opaque and sealed envelopes. When a baby was eligible for enrollment a new PIVC was inserted after obtaining written consent from one of the parents. Enrolled neonates were randomized to receive either heparin or normal saline, immediately before and after each dose of antibiotic. Babies in treatment group received 1.0 ml of heparinized saline containing 10 U ml−1 and babies in control group received 1.0 ml of normal saline.
The allotted medications were prepared every day and labelled as A or B by either of the two doctors who were not involved in the study. The code of A or B was disclosed only after completion of trial. Blinding was preserved by dispensing drugs and placebo in identical, indistinguishable containers. They were administered by a separately identified team of three resident doctors and one nurse. Care giving staff and parents were blinded to the group allocation. In neonates not requiring continuous IV fluids, one PIVC was inserted and used for administering antibiotics only. In neonates on continuous IV fluid infusion, a separate PIVC was used for administering antibiotics, so as to not interrupt the IV line. The catheter to be used for administering intermittent IV antibiotics was included in the study. A 24-gauge PIVC (NEOCAN Eastern Medikit Limited, Delhi, India) were used for catheterization in all neonates. Insertion and fixation of PIVC was done by trained resident doctor and nurses. Before the onset of the study, all healthcare personnel involved in insertion and fixation of catheter in the NICU were given demonstration on the technique of insertion and fixation of PIVC. Study medication was administered immediately after catheter insertion and then immediately before and after each antibiotics injection. Antibiotics were mostly administered at 8 or 12 hourly interval in both groups. The catheter site was monitored every 6 h for development of thrombophlebitis or any other sign of removal. The signs of removal were presence of any one of the following (i) Occlusion: defined as inability to infuse fluid. This was confirmed by resident doctor who attempted to push 1 ml of normal saline using a 5-ml syringe (ii) Phlebitis: defined as pain, swelling, erythema or indurations at the site of catheter with or without a palpable venous cord and (iii) Infiltration: infusate entering the subcutaneous tissue instead of the vein leading to swelling and difficulty in pushing antibiotics.
Platelet count was obtained at time of enrollment and then every third day till end of enrollment. Coagulation profile was done at the time of stopping study drug or if coagulation abnormality was suspected clinically. Study medication was stopped if neonates developed thrombocytopenia or coagulation defect or intracranial bleed and patients were analyzed by intention to treat analysis. Intracranial hemorrhage was recognized by ultrasound, which was obtained before enrollment on seventh day and at discharge from hospital.
Primary outcome variable was functional duration of first PIVC defined as the time between insertion and removal of the first catheter. Secondary outcome variable was average catheter duration of any catheter inserted, defined as total number of hours of catheterization divided by total number of catheterizations. Other outcomes analyzed were complications associated with PIVC including occlusion, phlebitis or infiltration, incidence of abnormal coagulation profile, allergic reaction to study medication (fever, high blood pressure, heart rate >160 min−1 , respiratory distress, skin rashes consisting of red spot), heparin-induced thrombocytopenia (HIT) defined as platelet count <15 0000 mm−3 after exclusion of other cause of thrombocytopenia.5, 6
We needed to enroll 56 subjects in each group to detect a 30% increase in functional duration of PIVC from baseline duration of 24±15 h (reported in a study from India)2 with 80% power and 95% confidence interval (CI). To account for some unforeseen loss of the cases, it was decided to randomize 120 babies in two equal groups.
All baseline and outcome data were recorded prospectively on a predesigned proforma. The data were checked daily for completion, consistency and accuracy. Analysis was performed using Stata version 11 (STATA 11.1, Stata Corp, College Station, TX, USA) by intention to treat. Categorical variables were compared with χ2-square test for discrete variable and Student t-test was used for continuous variables. Kaplan–Meier survival analysis was carried out to compare the rate of catheter change with time between the two groups. A P-value of <0.05 was considered significant.
Observation and Results
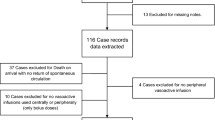
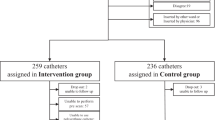
Of the 130 eligible neonates, 10 babies were excluded owing to refusal of consent. Remaining 120 neonates were randomized in to two groups of 60 neonates each (Figure 1). The two groups were comparable with respect to postnatal age, sex, birth weight, mode of delivery, nature of IV antibiotics, site of catheterization and person inserting the catheter (Table 1). Number of IV canula inserted in heparin group were 96 and 100 in saline group.
Primary outcome
There was a statistically significant increase in functional duration of first catheter in neonates in heparinized saline group as compared to normal saline group. Mean functional duration of first catheter was more in heparinized saline group, mean (s.d.) of 71.68 h (27.3) as compared with 57.7 h (23.6) in normal saline group (P<0.005). Between the two groups, mean difference (95% CI) in functional duration of catheter was 13.9 h (4.7–23.1) in favor of heparinized saline group (Figure 2). Kaplan–Meier survival estimate showed that at 90 h of duration, 7 first canula (10.0%) were patent in normal saline group compared with 17 first canula (25.0%) in heparinized saline group (P<0.001) ( Figure 2).
Secondary outcomes
Average catheter duration (of any catheters used on a baby) was more in heparinized saline group, with mean (s.d.) of 71.12 h (23.86) as compared with 57.29 h (18.66) in normal saline group (P<0.001). Between the two groups, mean difference (95% CI) in average catheter duration was 13.86 h (7.84–19.88) in favor of heparinized saline group (Figure 3). Kaplan–Meier survival analysis showed that 63.0%, 13.0% and 1.0% of the catheters on saline infusion were patent at 50, 75 and 90 h, respectively, as against 82.3%, 49.0% and 11.5% in the heparinized group at respective time points (Figure 3). These differences were statistically significant (P<0.001).
Occlusion at catheter site was commonest indication for catheter removal in both groups (77.3% vs 71.7%). Phlebitis was next most common cause (28 and 22% in two groups). There was no significant difference in abnormality in platelet count and coagulation profile, intracranial bleed and allergic reaction in either group.
Discussion
Our study demonstrated that use of heparinized saline flush (10 U ml−1) before and after intermittent IV antibiotics resulted in prolonged functional duration of 24-gauge peripheral IV catheter in neonates by almost 16 h. There exists equivocal evidence that use of heparin prolongs the duration of PIVC in neonatal age group. Mudge et al.4 had earlier demonstrated the effectiveness of heparin flush solutions in maintaining the patency of 24-gauge peripheral intermittent infusion devices. It was, however, a nonrandomized, sequential, but blinded study. The survival analysis in their study demonstrated that the median duration of catheter flushed with heparin was 42 h and with saline was 35.3 h (P=0.02). Danek et al.7 also reported that heparinized saline was more effective in prolonging duration of 24-gauge PIVC in neonates. Tayler et al.8 had demonstrated that heparin lock is probably better than continuous infusion fluids for prolongation of PIVC. In a cohort study using historical controls, Flint and Davies9 reported that intermittent flushing with heparin works as well as continuous infusion of heparin. A recent study by Pereza et al.10 reported that intermittent flushing with even normal saline was better than continuous infusion of normal saline at rate of 2 ml h−1 for prolonging duration of PIVC in newborns.
Contrary to above studies, Kotter et al.11 did not find significant prolongation of IV catheter patency with use of 10 U ml−1 of heparinized saline to flush the catheters. Similarly, Paisley et al.12 and Hanrahan et al.13 also did not demonstrate any increase in functional duration of PIVC with use of heparinized saline. A recent trial by Arnts et al.14 also failed to demonstrate any advantage of heparin solution when compared to normal saline flush in neonates. In addition, Schultz et al.15 reported decrease in duration of patency of PIVC with use of heparinized saline. Another recent trial by Cook et al.16 also reported that saline flushed PIVC lasts longer than heparinized saline flushed PIVC by average of 13 h. Cochrane systemic review reported inconclusive evidence for heparin use for IV catheters in neonates with two studies reporting benefit4, 17 and one study reporting harm,15 whereas two studies reporting no difference.11, 18 Paisley et al.12 reported that duration of patency was significantly longer in term, but not in preterm infants.
Increasing the concentration of heparin has been tried to increase the efficacy of heparinized saline to maintain the patency of IV catheter. In a randomized, double blinded trial on 90 neonates, Heilskov et al.18 demonstrated that increasing concentration of heparinized saline flush solutions (2 and 10 U ml−1), did not have any effect on the duration of IV catheter patency. A recent trial by Bertolino et al.19 in adult patients reported efficacy of 100 U ml−1 heparinized saline flush in reducing catheter related phlebitis/occlusion and number of catheter per patients. This study used maximum concentration of heparin tried in heparinized solutions but it did not demonstrate any increase in heparin related complications.
Most previous studies have used heparin flush only after IV antibiotics. However, our study used heparin flush both before and after IV antibiotics. This could be the reason for prolongation of fuctional duration of catheter patency in our study. Another strength of our study was the double blind trial, as many previous study were either open labeled or used historical controls.
From the above results, we can conclude that administration of 10 U ml−1 heparinized saline before and after IV antibiotics increases the functional duration of PIVC in neonates without any significant side effects.
References
Johnston CC, Stevens BJ . Experience in a neonatal intensive care unit affects pain response. Pediatrics 1996; 98: 925–930.
Dalal SS, Chawla D, Singh J, Agarwal RK, Deorari AK, Paul VK . Limb splinting for intravenous cannulae in neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2009; 94: 394–396.
Shah P, Ng E, Sinha A . Heparin for prolonging peripheral intravenous catheter use in neonates. Cochrane Database Syst Rev 2005; 3: CD002774.
Mudge B, Forcier D, Slattery MJ . Patency of 24 gauge peripheral intermittent device: a comparison of heparin and saline flush solutions. Pediatr Nurs 1998; 24: 142–145, 149.
Lesko SM, Mitchell AA, Epstein MF, Louik C, Giacoia GP, Shapiro S . Heparin use as a risk factor for intraventricular hemorrhage in low-birth-weight infants. N Eng J Med 1986; 314: 1156–1160.
Ahmed I, Majeed A, Powell R . Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J 2007; 83: 575–582.
Danek GD, Noris EM . Pediatric IV catheters: efficacy of saline flush. Pediatric Nursing 1992; 18: 111–113.
Taylor J, Shanon R, Kilbride H . Heparin lock intravenous line use in newborn infants: a controlled trial. Clinic Pediatr 1989; 28: 237–240.
Flint A, Davies M . The intravenous cannula for newborn infants requiring only intravenous medication: continuous infusion or intermittent flushing? J Infus Nurs 2008; 31: 346–349.
Pereza A, Feuza I, Brotschi B, Bernet V . Intermittent flushing improves cannula patency compared to continuous infusion for peripherally inserted venous catheters in newborns: results from a prospective observational study. J Perinat Med 2012; 40: 311–314.
Kotter RW . Heparin vs saline for intermittent intravenous device maintenance in neonates. Neonatal Netw 1996; 15: 43–47.
Paisley MK, Stamper M, Brown J, Brown N, Ganong LH . The use of heparin and normal saline flushes in neonatal intravenous catheters. Pediatr Nurs 1997; 23: 521–527.
Hanrahan KS, Kleiber C, Berends S . Saline for peripheral intravenous locks in neonates: evaluating change in practice. Neonatal Network 2000; 19: 19–24.
Arnts IJ, Heijnen JA, Wilbers HT, van der Wilt GJ, Groenewoud JM, Liem KD . Effectiveness of heparin solution versus normal saline in maintaining patency of intravenous locks in neonates: a double blind randomized controlled study. J Adv Nurs 2011; 67: 2677–2685.
Schultz AA, Drew D, Hewitt H . Comparison of normal saline and heparinized saline for patency of IV locks in neonates. Appl Nurs Res 2002; 15: 28–34.
Cook L, Bellini S, Cusson RM . Heparinized saline vs normal saline for maintenance of intravenous access in neonates: an evidence-based practice change. Adv Neonatal Care 2011; 11: 208–215.
Golberg M, Sankaran R, Givelichan L, Sankaran K . Maintaining patency of peripheral intermittent infusion devices with heparinized saline and saline. Neonatal Intensive Care 1999; 12: 18–22.
Heilskov J, Kleiber C, Johnson K, Miller J . A randomized trial of heparin and saline for maintaining intravenous locks in neonates. J Soc Pediatr Nurs 1998; 3: 111–116.
Bertolino G, Pitassi A, Tinelli C, Staniscia A, Guglielmana B, Scudeller L, Luigi Balduini C . Intermittent flushing with heparin versus saline for maintenance of peripheral intravenous catheters in a medical department: a pragmatic cluster-randomized controlled study. Worldviews Evid Based Nurs 2012; 9: 221–226.
Author information
Authors and Affiliations
Corresponding author
Additional information
This abstract was presented in annual meeting of Pediatrics Academic Society (PAS) held in BOSTON, USA(E-PAS2012:4524.320)
Rights and permissions
About this article
Cite this article
Upadhyay, A., Verma, K., Lal, P. et al. Heparin for prolonging peripheral intravenous catheter use in neonates: a randomized controlled trial. J Perinatol 35, 274–277 (2015). https://doi.org/10.1038/jp.2014.203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.203
This article is cited by
-
Heparin versus normal saline for the care of peripheral intravenous catheters in pediatrics: a meta-analysis of randomized controlled trials
BMC Pediatrics (2024)
-
Continuous infusion versus intermittent flushing: maintaining peripheral intravenous access in newborn infants
Journal of Perinatology (2016)