Abstract
JAK-STAT is a rational drug target in myelofibrosis (MF) given its association with JAK2/MPL mutations and aberrant inflammatory cytokine expression. We conducted a Phase 1/2 trial of CYT387, a potent JAK1/2 inhibitor, in patients with high- or intermediate-risk primary or post-polycythemia vera/essential thrombocythemia MF. Pre-planned safety and efficacy analysis has been completed for the initial 60 patients. In the dose-escalation phase (n=21), the maximum-tolerated dose was 300 mg/day based on reversible grade 3 headache and asymptomatic hyperlipasemia. Twenty-one and 18 additional patients were accrued at two biologically effective doses, 300 mg/day and 150 mg/day, respectively. Anemia and spleen responses, per International Working Group criteria, were 59% and 48%, respectively. Among 33 patients who were red cell-transfused in the month prior to study entry, 70% achieved a minimum 12-week period without transfusions (range 4.7–>18.3 months). Most patients experienced constitutional symptoms improvement. Grade 3/4 adverse reactions included thrombocytopenia (32%), hyperlipasemia (5%), elevated liver transaminases (3%) and headache (3%). New-onset treatment-related peripheral neuropathy was observed in 22% of patients (sensory symptoms, grade 1). CYT387 is well tolerated and produces significant anemia, spleen and symptom responses in MF patients. Plasma cytokine and gene expression studies suggested a broad anticytokine drug effect.
Similar content being viewed by others
Introduction
Myelofibrosis (MF) is a BCR-ABL1-negative myeloproliferative neoplasm that develops de novo (that is, primary MF, or PMF) or from antecedent polycythemia vera (PV; that is, post-PV MF) or essential thrombocythemia (ET; that is, post-ET MF). Cardinal clinical features include progressive anemia and/or splenomegaly, frequently associated with debilitating constitutional symptoms (fatigue, night sweats, bone pain, pruritus and cough) and weight loss.1 Based upon contemporary risk models,2 survival of PMF patients ranges from 1.8–17.5 years;1 similar data are not available for post-PV/ET MF. Approximately 15% of PMF patients are estimated to be suitable candidates for allogeneic stem cell transplantation based upon age and disease-risk considerations;1 the majority of MF patients are treated with conventional drugs where the therapeutic goal is symptom palliation.3
While multiple somatic mutations have been identified in MF, the disease-causing molecular lesion remains obscure.4 Regardless, given the relatively high frequency of JAK-STAT pathway-activating mutations in MF (JAK2V617F 60%,1 MPLW515 8%5) and recapitulation of many aspects of the disease in animal models expressing JAK2V617F,6 several JAK inhibitors are under study for MF therapy,7, 8 and one such drug (ruxolitinib) was recently approved for use in this indication.9, 10
We describe here interim results from a Phase 1/2 study of CYT387, an orally bioavailable JAK1/2 inhibitor that has shown activity in pre-clinical myeloproliferative neoplasm models,11, 12 for MF treatment.
Patients and methods
Study oversight
The protocol was approved by the Mayo Clinic institutional review board and conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
Trial design and treatment
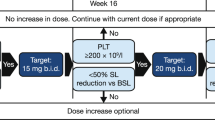
This open label, non-randomized study was registered at clinicaltrials.gov (CCL09101; NCT00935987) and was conducted in two phases: the focus of this report is the single-center (Mayo Clinic) dose-escalation phase with supernumerary patient addition (Part 1) to determine the safety and tolerability of CYT387, and to identify a therapeutic dose for the confirmation phase. All patients treated in Part 1 have completed the core study (that is, 9 cycles). A multiple-center dose-confirmation phase (Part 2), which enrolled an additional 106 patients, was subsequently initiated with cohort expansion at or below the maximum-tolerated dose of CYT387; analysis of these data are ongoing. Other primary end-points for Part 1 included the assessment of pharmacokinetic behavior and therapeutic efficacy of CYT387, as measured by International Working Group for Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) criteria.13 CYT387 capsules were administered orally once daily with a treatment plan for continuous daily therapy for 36 weeks (9 × 28-day cycles). Intra-patient dose escalation was permitted after completion of at least three cycles at the starting dose. Treatment beyond nine cycles was permitted on an extension study (CCL09101E; NCT01236638) if deemed beneficial to the patient and if well tolerated.
Eligibility criteria
Patients were eligible if they were ⩾18 years of age with PMF, post-PV MF or post-ET MF according to the 2008 World Health Organization (WHO) criteria,14 and intermediate-2 or high-risk disease per the International Prognostic Scoring System (IPSS)15 or intermediate-1 risk disease with either symptomatic hepatosplenomegaly or unresponsiveness to available therapies. Other eligibility criteria included an Eastern Cooperative Oncology Group performance status of ⩽2, neutrophil count of 0.5 × 109/l or more, platelet count of 50 × 109/l or more, and absence of peripheral neuropathy of grade 2 severity or greater.
Assessment of toxicity and response
Safety assessments were performed weekly during cycle 1, every other week during cycles 2 and 3, and every 4 weeks thereafter. Toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Responses were measured every 4 weeks per IWG-MRT criteria.13 Palpable spleen and liver measurements were recorded and patient-reported disease symptoms were assessed and graded (mild, moderate or severe) by the investigator at every study visit. A ⩾50% improvement in symptoms relative to baseline was characterized as ‘marked improvement’. Bone marrow sampling was planned at the end of every third cycle and at the end of cycle 9, only for those patients whose peripheral blood response potentially qualified as having achieved complete remission (CR) per IWG-MRT criteria.13 JAK2V617F allele burden in the granulocyte fraction of peripheral blood was measured as previously described.16
Cytokine assessment and gene expression profiling
Plasma samples for cytokine measurement were collected at baseline and at the end of cycle 3. Concentrations of 30 cytokines were analyzed in duplicate by using Multiplex Bead-Based Luminex technology (Invitrogen, Carlsbad, CA, USA), as previously described.17 RNA from peripheral blood mononuclear cells was profiled on microarrays (Human HT-12 v4, Illumina, San Diego, CA, USA); gene expression data analysis (Genomics suite v6.6, Partek, St Louis, MO, USA; Genesifter, Geospiza-Perkin Elmer, Seattle, WA, USA) and gene pathway analysis (MetaCore software, GeneGo Inc., St Joseph, MI, USA) were performed using the manufacturer’s instructions. Microarray data are deposited in Gene Expression Omnibus repository (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE40841).
Results
Patients and dose schedules
A total of 60 patients were enrolled in Part 1 of the study; baseline clinical characteristics are shown in Table 1. Seventy percent of patients were JAK2V617F-positive and 80% had a palpable spleen size of >10 cm. The median hemoglobin level was 9.4 g/dl (range 7.1–14.4), and 33 patients (55%) were transfusion-dependent at baseline by IWG-MRT criteria.13 Eleven (18%) and 10 patients (17%) had previously received JAK inhibitor (ruxolitinib or SAR302503) or IMiD (thalidomide, lenalidomide and/or pomalidomide) therapy, respectively.
In the dose-escalation phase, the starting dose of CYT387 was 100 mg/day; subsequent dose levels were 150, 200, 300 and 400 mg/day. At 400 mg/day, two of six patients experienced dose-limiting toxicity; consequently the maximum-tolerated dose was declared at 300 mg/day. As clinical responses were observed at 150 and 300 mg/day dose levels, both were declared as ‘biologically effective’, and an additional 18 and 21 patients were accrued at 150 mg/day (total=21) and 300 mg/day (total=27), respectively (Table 1).
Efficacy
Anemia
A total of 41 patients (68%) were evaluable for anemia response based on a baseline non-transfused hemoglobin level of <10 g/dl (n=8) or transfusion dependency at baseline (n=33) (Table 2). Overall, 23 (70%) of the previously transfusion-dependent patients achieved transfusion-independence by IWG-MRT criteria.13 With the use of more stringent criteria (that is, requiring a minimum non-transfused hemoglobin level of 8.0 g/dl during the 12-week assessment period), the response rate (67%) was essentially unchanged. The median duration of transfusion-independence at data cutoff was 9.6 months (range 4.7–18.3), with responses still ongoing. Only one of eight non-transfusion-dependent anemic patients achieved anemia response by IWG-MRT criteria. Overall, 24 patients (59%) achieved ‘clinical improvement’ response by IWG-MRT criteria based upon anemia improvement. Transfusion-independence responses were not significantly different at the starting dose of 300 mg/day (68%) versus 150 mg/day (50%) (P=0.3).
Spleen size
Fifty-two patients were evaluable for spleen response by IWG-MRT criteria. Overall, 25 patients (48%) achieved a spleen response; the maximal change in palpable spleen size by starting dose is shown in the Figure 1. The mean change in spleen size from baseline per treatment cycle is shown in Supplementary Table 1; spleen responses were rapid and durably maintained throughout the treatment period. There was no difference in spleen response (50%) when comparing patients starting treatment at 300 mg/day versus 150 mg/day.
Shows the maximum percentage decrease in palpable spleen size during the core study for the 52 patients (coded by starting dose) evaluable for spleen response by IWG-MRT criteria.13 ‘Clinical improvement’ response by these criteria is either a minimum 50% reduction in palpable splenomegaly of a spleen that is at least 10 cm at baseline, or a spleen that is palpable at more than 5 cm at baseline becomes not palpable, with the size reduction persisting for a minimum 8-week period.
Patient-reported symptoms
The following symptoms were recorded at baseline (severity scale of mild/moderate/severe by physician assessment): pruritus, 16 patients (prevalence, 27%; severity, 6/63/31%); night sweats, 29 patients (prevalence, 48%; severity, 3/49/48); cough, 5 patients (prevalence, 8%; severity, 0/20/80%); bone pain, 19 patients (prevalence, 32%; severity, 16/63/21%); fever, 7 patients (prevalence, 12%; severity, 71/29/0%); and appetite loss, 10 patients (prevalence, 17%; severity, not recorded). Significant improvement in the aforementioned symptoms was recorded with study treatment (Table 3). Rates of complete resolution of symptoms after 1 and 3 months of treatment were as follows: pruritus, 63/75%; night sweats, 59/79%; cough, 20/20%; bone pain, 58/63%; fever, 86/100%; and appetite loss, 30/40%. Symptom responses were durable over the core study period. A 3.0-kg increase in median weight from 74 kg at baseline to 77 kg at the end of the core study was observed across dose levels.
Bone marrow histology
None of the patients had a peripheral blood response that qualified as CR; consequently, follow-up bone marrow biopsies were not routinely performed.
Pharmacokinetics
Dose-linear Cmax and exposure (area under curve) were observed between the 150 and 300 mg/day doses, with mean elimination T1/2 at steady state ranging from 3.9–6.1 hours (Supplementary Figure 1).
Safety and adverse events
Dose-limiting toxicities were grade 3 headache and asymptomatic grade 3 hyperlipasemia in one patient each that were reversible upon temporary drug discontinuation. Overall, 52 patients (87%) completed the core study (9 cycles). Reasons for discontinuation were adverse event (AE) (n=6, only one AE attributed to CYT387) and lack of response (n=2) (Supplementary Table 2). Five patients died during the core study (two patients with respiratory failure and one each with restrictive cardiomyopathy, gastrointestinal hemorrhage and intracranial hemorrhage); none of the deaths were attributed to CYT387. Nine serious adverse events (SAEs) in six patients were attributed to CYT387 during the core study (headache=2, increased serum lipase=2, thrombocytopenia=1, neutropenia=1, increased serum alanine aminotransferase=1, increased serum aspartate aminotransferase=1 and hypertension=1) (Supplementary Table 3); a full listing of treatment-emergent SAEs is presented in Supplementary Table 4.
At data cutoff (14 November 2011), after a median follow-up of 15.8 months (range 2.9–25.5), 41 patients (68%) were receiving CYT387 treatment. Treatment-related AEs at least possibly related to CYT387 are shown in Table 4. Non-hematologic grade 3/4 AEs were limited to increased aspartate aminotransferase (3%), alanine aminotransferase (3%), headache (3%) and hyperlipasemia (5%). Dizziness (predominantly grade 1) was seen in one-quarter of patients; it generally occurred within the first hour of initiating CYT387 therapy and resolved within a few hours. No clear dose-dependence was observed. Rarely, mild intermittent dizziness persisted for 2–3 weeks; no patient discontinued treatment due to this AE.
Sixteen patients (27%) reported new-onset peripheral neurologic symptoms (n=13) or worsening of pre-existing symptoms (n=3) attributed to the study drug; these were almost exclusively grade 1 hypoesthesia/paresthesias in the digits/extremities. Six of these patients (38%) had been previously exposed to IMiD or JAK inhibitor treatment. The median time to emergence of peripheral neuropathy was 141 days; at the time of data cutoff, one patient had discontinued treatment and five patients (31%) had been dose-reduced due to peripheral neuropathy. Half of the affected patients (n=8) had complete resolution of neurological symptoms at last assessment despite continuing CYT387 therapy. No clear dose-dependence was identified.
Gastrointestinal AEs (nausea 18%, diarrhea 13%) were mild in severity (grade 1–2) and self-limited; no dose reduction or drug discontinuation was needed.
The most frequent treatment-related hematological AE was thrombocytopenia (32% grades 3/4), although for patients with baseline platelet counts of >100 × 109/l, only nine (21%) experienced grade 3/4 thrombocytopenia. Treatment-related anemia and neutropenia were relatively infrequent: two patients (3%) and three patients (5%) with grade 3/4 severity, respectively.
Of the 48 patients in the 150 mg/day and 300 mg/day cohorts, 16 patients (33%) required dose reduction for any cause (Supplementary Table 5). The frequency of such events was higher in the latter group; however, no clear pattern for a specific recurrent AE emerged in this analysis. At data cutoff, no new safety findings have emerged with CYT387 dosing beyond nine cycles of therapy.
Correlative studies
Plasma cytokines
Paired pre- and post-treatment plasma samples were available for 41 patients; the overall change in cytokine levels (Supplementary Table 6) as well as the proportion of patients with decreased versus increased cytokine levels at end of cycle 3 relative to baseline (Supplementary Table 7) was analyzed. A change in interleukin (IL)-1 receptor antagonist (IL-1RA) (P=0.008) and IL-1β (P=0.03) levels was associated with transfusion-independence response. Similarly, spleen response by IWG criteria was significantly associated with changes in: IL-1RA (P=0.04), IL-1β (P=0.005), IL-2 (P=0.001), fibroblast growth factor-basic (P=0.02), tumor necrosis factor-α (0.03), and macrophage inflammatory protein-1β (0.03). This univariate analysis considered cytokine levels as a continuous variable; there was a treatment-associated decrease in levels of cytokines associated with anemia and/or spleen response.
JAK2V617F allele burden
Paired allele burden data were available for 40 of 42 JAK2V617F-positive patients. The median (range) allele burden at baseline and last available measurement during the core study was 22.5% (1–68%) and 14% (0–90%), respectively (paired-sign test P-value=0.2). For 23 patients with a baseline allele burden of ⩾10%, 6 and 3 patients had a minimum 50% decrease (median 30%, range 17–63%) and increase (median 27%, range 19–47%), respectively.
Gene expression profiling
Seventeen patients were studied based on sample availability; the initial pair-wise analysis identified a cluster of genes that were significantly downregulated with treatment (Supplementary Figure 2A; green spheres). Pathway enrichment analysis revealed association of these genes with cytokine regulation of immune response, cell proliferation and chemotaxis. A subsequent analysis compared gene expression profiles of anemia-responders versus non-responders; this identified a dominant cluster of overexpressed genes in responders (Supplementary Figure 2B; red spheres). Similar pathway analysis revealed enrichment for genes involved in immune system function with overlap between the two clusters (for example, IL-12β, IL-17, IL-21, IL-22 and IL-23) (Supplementary Table 8).
Discussion
The current study illustrates the scope of clinical benefit with CYT387 treatment in intermediate- or high-risk MF. In particular, the observed anemia response was unprecedented and, if confirmed, exceeds the anemia response seen with other MF therapies. Further, the responses in splenomegaly and constitutional symptoms were frequent, rapid in onset, durable during treatment and, although not directly comparable, appeared to be of similar magnitude to other JAK inhibitors.9, 10, 18, 19 Both starting doses chosen for the dose-expansion phase (that is, 150 and 300 mg/day) had significant activity; while there was a trend for more frequent anemia responses at 300 mg/day, spleen responses were equal at the two doses.
Overall, CYT387 was well tolerated, which is reflected in the favorable study retention rate (87%). Consistent with the aforementioned anemia response, the incidence of treatment-emergent anemia was exceedingly low; the latter is a significant problem with other JAK inhibitor drugs.9, 10, 18, 19 Thrombocytopenia and asymptomatic hyperlipasemia were noted; these are drug-class related AE’s; however, notably, no study discontinuation resulted from these findings. A unique aspect of CYT387’s AE profile is the occurrence in some patients of transient mild dizziness with or without mild hypotension after the first dose. No treatment was required; we managed this problem by holding antihypertensive medications on the first day of CYT387 treatment and encouraging liberal fluid intake. A mild sensory neuropathy either emerged or worsened during treatment in roughly one-quarter of patients; however, the neuropathy completely resolved in half of the affected patients during follow-up, despite ongoing CYT387 therapy. Only 38% of patients with neuropathy required dose reduction or treatment discontinuation. No clear dose or temporal dependency was noted. Given that ∼40% of affected patients had prior IMiD or JAK inhibitor therapy and symptoms of sensory peripheral neuropathy are commonly reported in MF patients,20 it will be important to objectively measure nerve function in a larger patient group without pre-existing neuropathy or risk factors for the same (for example, diabetes, prior exposure to neurotoxic drugs), to determine the true incidence of CYT387-related neuropathy.
The mechanism of anemia response to CYT387 therapy in MF is unclear. Our data suggests a cytokine-mediated effect; a majority of patients had treatment-related decrease in circulating IL-1β and IL-1RA levels, which were the only two cytokines associated with transfusion-independence response. Similarly, spleen response was correlated with treatment-associated decreases in a number of cytokines. Overall, these data implicate downregulation of circulating inflammatory cytokines, further confirmed by gene expression analysis, as the major mechanism for CYT387’s clinical activity in MF. While a similar global effect on cytokines has been reported for another JAK1/2 inhibitor, namely ruxolitinib,19 important differences in the clinical profile of the two drugs (for example, anemia response) belie such a superficial analysis. Perturbation of specific cytokines or particular immunocellular compartment(s) may underpin differences in the clinical activity of the two drugs.
In conclusion, this study shows clinical responses with CYT387 treatment in a significant proportion of MF patients. In contrast to other JAK inhibitors, treatment with CYT387 induced a substantive anemia benefit including transformation to transfusion-independence. Responses in splenomegaly and constitutional symptoms at least comparable with other inhibitors were also observed. These hematological, splenic and symptom responses satisfy the hitherto unmet clinical needs in MF patients. The current data, supplemented by data from the ongoing dose-confirmation phase and extension study, provide the basis for a controlled Phase 3 randomized trial.
References
Tefferi A, Lasho TL, Jimma T, Finke CM, Gangat N, Vaidya R et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc 2012; 87: 25–33.
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011; 29: 392–397.
Tefferi A . How I treat myelofibrosis. Blood 2011; 117: 3494–3504.
Tefferi A . Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 2010; 24: 1128–1138.
Pardanani A, Guglielmelli P, Lasho TL, Pancrazzi A, Finke CM, Vannucchi AM et al. Primary myelofibrosis with or without mutant MPL: comparison of survival and clinical features involving 603 patients. Leukemia 2011; 25: 1834–1839.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010; 17: 584–596.
Tefferi A . Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N Engl J Med 2012; 366: 844–846.
Tefferi A . JAK inhibitors for myeloproliferative neoplasms: clarifying facts from myths. Blood 2012; 119: 2721–2730.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: 787–798.
Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A . CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009; 23: 1441–1445.
Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood 2010; 115: 5232–5240.
Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood 2006; 108: 1497–1503.
Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood 2007; 110: 1092–1097.
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113: 2895–2901.
Kittur J, Knudson RA, Lasho TL, Finke CM, Gangat N, Wolanskyj AP et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer 2007; 109: 2279–2284.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A . Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 2011; 29: 1356–1363.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 2011; 29: 789–796.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011; 118: 401–408.
Acknowledgements
The study was funded by YM BioSciences who also provided the investigational drug.
Author contributions
The sponsor designed the study and jointly oversaw conduct of the study with their designee (Linda M Bavisotto, MD, Porta Clinica LLC, Seattle, WA, USA). Data were collected by the investigators and monitored by the sponsor. The investigators and sponsor jointly analyzed and interpreted the data. The first and last authors wrote the initial draft of the manuscript. All the authors and the sponsor reviewed and contributed to subsequent drafts and provided approval for submission of the final draft for publication.
Disclaimer
The corresponding author, Dr Ayalew Tefferi, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AP and AT have served as Principal Investigator/Co-investigator for clinical trials that received institutional support (free drug and funding) from Incyte, Sanofi-Aventis, TargeGen, YM BioSciences, Cytopia, Bristol-Myers Squibb, Celgene and Novartis. TargeGen and Cytopia have also provided support for laboratory studies relevant to clinical trials. MK is an employee of and owns stock in YM BioSciences Inc. All other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Pardanani, A., Laborde, R., Lasho, T. et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 27, 1322–1327 (2013). https://doi.org/10.1038/leu.2013.71
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.71
Keywords
This article is cited by
-
Momelotinib for myelofibrosis: our 14 years of experience with 100 clinical trial patients and recent FDA approval
Blood Cancer Journal (2024)
-
The application of JAK inhibitors in the peri-transplantation period of hematopoietic stem cell transplantation for myelofibrosis
Annals of Hematology (2024)
-
Determinants of survival and retrospective comparisons of 183 clinical trial patients with myelofibrosis treated with momelotinib, ruxolitinib, fedratinib or BMS- 911543 JAK2 inhibitor
Blood Cancer Journal (2023)
-
Momelotinib: First Approval
Drugs (2023)
-
Momelotinib: an emerging treatment for myelofibrosis patients with anemia
Journal of Hematology & Oncology (2022)