Abstract
Rearrangements of anaplastic lymphoma kinase (ALK) gene in non-small cell lung cancer (NSCLC) define a molecular subgroup of tumors characterized clinically by sensitivity to ALK tyrosine kinase inhibitors such as crizotinib. Although ALK rearrangements may be detected by reverse transcriptase-PCR, immunohistochemistry or fluorescence in situ hybridization (FISH), the optimal clinical strategy for identifying ALK rearrangements in clinical samples remains to be determined. We evaluated immunohistochemistry using three different antibodies (ALK1, 5A4 and D5F3 clones) to detect ALK rearrangements and compared those with FISH. We report the frequency and clinicopathologic features of lung cancers harboring ALK translocations in 594 resected NSCLCs (470 adenocarcinomas; 83 squamous carcinomas, 26 large cell carcinomas and 15 other histological subtypes) using a tissue microarray approach. We identified an ALK gene rearrangement in 7/594 cases (1%) by FISH and all anti-ALK antibodies correctly identified the seven ALK-positive cases (100% sensitivity), although the intensity of staining was weak in some cases. These data indicate that the use of antibodies with high sensitivity and avidity to ALK may provide an effective pre-screening technique to complement the more expensive and labor-intensive approach of ALK FISH testing.
Similar content being viewed by others
Main
Lung cancer remains the leading cause of cancer mortality worldwide.1 Non-small cell lung cancer (NSCLC) accounts for over 85% of lung cancer and is associated with 5-year survival rates of 15%.2 This reflects the small proportion of patients that present with potentially curable early stage disease, as well as the poor outcomes with conventional chemotherapy or radiotherapy for the majority of patients who present with advanced disease. Recognition of the molecular heterogeneity of NSCLC has led to the identification of molecular subgroups. The clinical benefit of identifying and targeting molecular drivers in NSCLC was first evident in the case of tumors with activating mutations in the epidermal growth factor receptor (EGFR) gene where seven randomized studies have shown the superiority of EGFR tyrosine kinase inhibitors over chemotherapy (reviewed in refs 3, 4, 5). Recently, rearrangements in the anaplastic lymphoma kinase gene (ALK) have been identified in a subgroup of NSCLC which have proved to be exquisitely sensitive to therapy with ALK tyrosine kinase inhibitors.
ALK is a member of the insulin family of receptor tyrosine kinases, and is typically expressed at low levels in regions of the central nervous system.6 ALK may be activated in cancer through multiple mechanisms including rearrangements, as is the case in anaplastic large cell lymphoma and inflammatory myofibroblastic tumor or through mutation and amplification as in neuroblastoma (reviewed in ref 7). In NSCLC, ALK activation is typically caused by a chromosomal rearrangement that results in a fusion of the 3′ kinase domain of ALK with various truncated portions of the (N-terminal) echinoderm microtubule-associated protein-like 4 (EML4) gene and its associated promoter.8, 9 EML4, is a cytoplasmic protein necessary for correct microtubule formation, belonging to the family of echinoderm microtubule-associated protein-like proteins.10, 11 The oncogenic activity of EML4–ALK comes from ligand-independent dimerization and subsequent constitutive activation of the ALK kinase domain with subsequent effects on proliferation, migration and survival.12 Mouse models where EML4–ALK is expressed specifically in lung epithelial cells, develop hundreds of adenocarcinoma nodules in both lungs soon after birth, which are eradicated following administration of an ALK inhibitor.13, 14 Other less common 5′ fusion partners have been described including kinesin family member 5B (KIF5B), TRK-fused gene (TFG) and kinesin light chain 1 (KLC1).8, 15, 16
ALK rearrangements have been reported to occur at frequencies that range from 0.4 to 15% in NSCLC,7, 17 although some series include selected patient populations likely to be enriched for ALK rearrangements. Most series, however, report an incidence of around 3–4% in unselected NSCLC populations. EML4–ALK rearrangements occur in adenocarcinomas that often show solid, acinar or signet ring histologic patterns.18, 19 ALK rearrangements have been commonly reported in patients who are younger and never or light smokers and are generally wild type for EGFR and KRAS mutations.20, 21 However, the clinical and pathologic features of ALK-positive NSCLC overlap significantly with ALK-negative NSCLC.
NSCLC tumors with ALK rearrangements demonstrate marked sensitivity to treatment with ALK tyrosine kinase inhibitors. In clinical trials with crizotinib in NSCLCs with ALK rearrangement partial response rates of 51–61% were seen in association with rapid symptom relief and improved quality of life leading to approval of crizotinib for this indication by the US FDA in August 2011.22 A recently reported phase III study has demonstrated a doubling of progression-free survival compared with standard chemotherapy in previously treated ALK-positive NSCLC.23 The activity of crizotinib in ALK-positive NSCLC highlights the need to test for and identify ALK rearrangements to select appropriate patients for ALK-directed therapies.
ALK fluorescence in situ hybridization (FISH) is currently the gold standard method and is the only FDA-approved method for detecting ALK rearrangements. However, because of the low frequency of ALK rearrangements and the high incidence of NSCLC, routine testing for ALK-positive tumors using only this method may not be feasible, because of cost, required expertise and the labor-intensive nature of performing and interpreting the test. Immunohistochemistry is potentially a useful technique to pre-screen cases for ALK FISH testing, reducing the burden of FISH testing to a manageable number. We evaluated the feasibility and validity of using immunohistochemistry for large scale pre-screening of NSCLC tumors to complement FISH testing. We present a large-scale multicenter study of ALK testing in resected lung cancers comparing three different commercially available ALK immunohistochemistry antibodies in comparison with ALK FISH using a break-apart probe.
Materials and methods
Patient Cohorts
Two cohorts of tissue microarrays constructed from surgical resections that were formalin fixed and paraffin embedded were utilized to test for ALK rearrangement. Lung adenocarcinoma tissue samples were obtained from two hospitals within the Sydney Local Heath District: Royal Prince Alfred Hospital and Concord Repatriation General Hospital, Sydney, Australia. The Sydney Local Health District cohort consisted of 279 patients with lung adenocarcinomas resected between 1990 and 2008 as previously described.24, 25, 26 The second cohort consisted of NSCLCs from two Melbourne hospitals: St Vincent’s Hospital, Melbourne, and the Peter MacCallum Cancer Centre, Melbourne, Australia. The St Vincent's Hospital-Peter MaCallum Cancer Centre cohort included 362 cases of NSCLCs of all histological types resected between 1996 and 2009 (including 223 adenocarcinomas). Thus, a total of 641 cases across the four centers were included in the study. EGFR and KRAS mutation testing was performed on all the ALK FISH-positive cases. Ethics approval was provided by the ethics review committees of the respective institutions (X10-0278; CH62/6/2004-116; HREC/10/RPAH/491; PMCC 03/90 and SVH A03/12).
FISH for ALK
All cases had interphase FISH performed for ALK rearrangement using the Vysis LSI ALK Dual Colour, Break-Apart Rearrangement Probe (Abbott Molecular, IL, USA). This probe detects rearrangements in 2p23 encompassing the ALK gene, which includes a SpectrumOrange labeled 250 kb DNA fragment telomeric to ALK (3′ end) and a SpectrumGreen labeled 300 kb DNA fragment centromeric to ALK (5′ end). The probe set does not identify the specific rearrangement gene partner. Tissue microarray sections cut at 3 μm were stained for ALK FISH according to the manufacturer’s instructions. Signals were counted in at least 50 tumor nuclei per case using an epifluorescence microscope (Zeiss, Oberkochen, Germany). FISH for ALK locus rearrangement was considered positive if at least 15% of cells analyzed showed either a split of one set of red and green signals greater than two signal widths apart, and/or if loss of one green signal (5′ probe) had occurred27, 28, 29, 30, 31 as per Abbott Molecular scoring criteria. A positive control consisting of an independently validated lung tumor confirmed by FISH to be positive for ALK rearrangement was included. Negative controls included an independently validated lung tumor confirmed to be FISH negative for rearrangement and non-tumor lung tissue.
Immunohistochemistry for ALK
Immunohistochemistry was performed on TMA sections, cut at 4 μm, using three different antibodies to ALK. The Dako mouse monoclonal ALK1 antibody CD246 (Clone ALK-1, M7195, Dako, Glostrup, Denmark) was used at 1:50 dilution overnight at 4 °C and performed manually with the Envision FLEX+ Mouse (LINKER) detection system (Dako). Heat-induced epitope retrieval was performed using pH 9.0 buffer (Dako) in a pressure cooker for 2 min at 124 °C. The Novocastra mouse monoclonal antibody p80 ALK (Clone 5A4, NCL-ALK, Leica, Wetzlar, Germany) was used at 1:25 dilution for 52 min. Staining was performed using the UltraView DAB universal detection kit (Roche, Basel, Switzerland) including an Amplification Kit (Roche), and was performed on a Benchmark ULTRA autostainer (Roche). The Cell Signaling Technology rabbit monoclonal ALK XP (Clone D5F3, 3633P, Cell Signaling Technology, Danvers, MA, USA) was used at 1:100 dilution for 24 min. Staining was performed using the OptiView DAB Immunohistochemistry Detection kit (Roche) with an Amplification kit (Roche). Positive controls included lung tumor confirmed by FISH to be positive for ALK rearrangement. Negative controls included lung tumor confirmed by FISH to be negative for rearrangement as well as non-tumor lung tissue. Expression of all ALK antibodies on each tissue microarray tissue section was assessed and scored independently by two experienced pulmonary pathologists (WC and PAR) blinded to clinicopathologic information and the results of ALK FISH. Each pathologist scored each TMA tumor core semiquantitatively by determining the percentage of tumor cells with cytoplasmic staining and the intensity of cytoplasmic staining, as follows: 1+, weak/faint cytoplasmic staining; 2+, moderate cytoplasmic staining; and, 3+, intense cytoplasmic staining. Cases with 1+, 2+ and 3+ staining were regarded as positive and cases with no staining were regarded as negative. In cases with a discrepancy in immunohistochemistry scoring, two pathologists reviewed the cases in conference and a consensus score was established.
Statistical Analysis
Clinicopathological features were compared using Mann–Whitney U-test for continuous variables and Fisher’s exact test for binomial variables using IBM SPSS statistics version 21 (IBM, Chicago, IL, USA). P-values <0.05 were considered significant.
Results
Clinical and pathological characteristics of the cohorts studied are shown in Table 1. Of the 641 cases analyzed, 47 cases (7%) were lost because of missing tumor cores or non-tumor tissue only in cores within tissue microarrays, culminating in complete overlapping results for ALK FISH and all three ALK antibodies for 594 cases (Supplementary Diagram 1). Missing results included 30 missing FISH results and an additional 17 missing immunohistochemistry results.
ALK FISH
ALK rearrangement detected by FISH was observed in 7/594 (1%) of the combined cohort with 3/279 (1%) cases in the Sydney Local Health District tissue microarrays and 4/315 (1%) cases in the St Vincent's Hospital-Peter MacCallum Cancer Centre tissue microarrays (Table 2). Among pure adenocarcinomas, 6/478 (1%) showed an ALK rearrangement. One of the FISH-positive cases showed a predominant signal pattern of deletion of the 5′ region (nuclei with a single red signal in addition to a fused signal), whereas the remaining six cases showed a break-apart signal pattern, where one fusion signal and a single red and green signal pattern was observed. A further case showed a subtle split signal pattern that was <2 signal distances in separation and thus categorized as technically FISH negative, as per Abbott Molecular scoring criteria.27, 32
ALK Immunohistochemistry
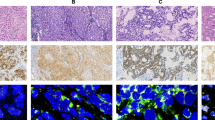
Of the 594 cases assessable by immunohistochemistry, cytoplasmic expression of ALK was observed in 13/594 (2%) cases with the ALK1 antibody (Dako), 18/594 (3%) cases with the 5A4 antibody (Novocastra) and 13/594 (2%) cases with the D5F3 antibody (Tables 2 and 3; Figure 1). All other cases as well as normal bronchus and peripheral lung tissue showed no immunoreactivity. All three antibodies stained ALK rearranged cases (100% sensitivity; Table 4) as well as 6–11 additional cases that were ALK negative that varied with different antibodies. The specificity was high among all three antibodies (98–99%), however, positive predictive values were 39% for 5A4, and 54% for ALK1 and D5F3 (Table 4). Negative predictive values were 100% for all three antibodies. The 5A4 and D5F3 clone antibodies produced stronger staining intensity of ALK rearranged cases compared with the ALK1 antibody (Table 3 and Figure 1) with D5F3 showing strong 3+ staining in 4/7 FISH-positive cases with less background staining than the other antibodies while ALK1 showed mostly weak staining. However, the intensity of staining ranged from very weak to strongly positive even with the 5A4 and D5F3 clones (Table 3). Heterogeneous staining was noted among multiple TMA cores from the same case, ranging from 1+ staining to negative in some cores. The one case that was technically FISH negative but showed subtle signal separation, displayed immunoreactivity with all three ALK antibodies, with staining ranging from 1 to 2+. One ALK FISH-negative case displayed very focal 3+ (8%) staining with the D5F3 antibody but was negative with the other antibodies.
Representative staining for ALK immunohistochemistry using ALK-1 clone antibody (a, b), 5A4 clone antibody (c, d) and D5F3 clone antibody (e, f) and for ALK FISH staining (g, h; arrows show positive and negative signal patterns). ALK rearrangement positive cases (a, c, e, g) and ALK rearrangement negative cases (b, d, f, h) are shown.
Clinical and Histological Characteristics of ALK-Positive Tumors
The seven ALK immunohistochemistry and FISH-positive cases consisted of three acinar predominant adenocarcinomas, two papillary predominant adenocarcinomas, one minimally invasive adenocarcinoma and one pleomorphic carcinoma (comprising 60% solid predominant adenocarcinoma and 40% giant cell carcinoma). Focal signet ring cell morphology was seen in three of the cases and a focal cribriform pattern was seen in four cases. Four of the six pure adenocarcinoma cases showed co-expression of TTF1 and TP63, with strong and diffuse TTF1 staining and weak-to-moderate patchy nuclear TP63 staining. The other two pure adenocarcinomas expressed TTF1 but lacked TP63. The pleomorphic carcinoma showed no expression with either TTF1 or TP63, but showed positive staining with alcian blue-PAS/D indicative of mucin in the solid adenocarcinoma component. The case that showed a narrow, technically negative split signal and ALK immunohistochemistry positivity displayed positive TTF1 staining and was negative for TP63 staining.
ALK rearranged cases were typically younger (P<0.002) and more likely to be female (P=0.015, Fisher’s exact test). Four of the ALK rearranged cases were never-smokers (57%), two cases had a history of smoking (29%) and one case had unknown smoking status. All ALK rearranged cases were wild type for EGFR and KRAS.
Discussion
Identification of ALK rearrangements in patients with NSCLC is critical to direct patients to therapy with highly effective ALK tyrosine kinase inhibitors such as crizotinib. Although there are three major methodologies to identify ALK rearrangements in clinical tumor samples including FISH, immunohistochemistry and reverse transcription-PCR, FISH has been the method used to identify patients for clinical trials with ALK inhibitors. However, FISH suffers from a number of limitations and when used as a single modality to screen large populations may not represent the most efficient approach from a logistic or cost-effectiveness perspective.33 In this context, we present the largest multi-center study to date to assess ALK rearrangement in NSCLC evaluating immunohistochemistry with three different ALK targeting antibodies including the ALK1, 5A4 and D5F3 clone immunohistochemistry antibodies in comparison with FISH.
These data reveal that ALK rearrangements occurred in a small subset (approximately 1%) of lung adenocarcinomas (or NSCLC with an adenocarcinoma component) in an Australian population with resected lung cancer. Although ALK rearrangements have been reported to occur in a range of 0.4–15% of NSCLC,7, 17 the frequency of ALK rearrangements in comparable populations from the United States (8/305;3%)21 and Canada (3/273;1%)34 is similar suggesting an ascertainment bias in many other series. However, we cannot exclude geographical variations or differential incidence in early vs advanced disease as an explanation for the low frequency of ALK rearrangements observed in our cohort.
When high-affinity antibodies are used with a highly sensitive detection method, ALK immunohistochemistry may provide an effective pre-screening technique to complement ALK FISH testing. If used in a routine setting, ALK immunohistochemistry could reduce the number of cases requiring ALK FISH testing, which is a more expensive and time consuming technique that requires considerable expertise. If ALK immunohistochemistry using the 5A4 antibody was undertaken, for every 100 adenocarcinomas requiring determination of ALK status, FISH testing would only be required in three cases (3%), of which one would be expected to be positive. The time saved to perform and interpret three FISH tests vs 100 would also be substantial. Other advantages of using immunohistochemistry as a screening tool include the detection of dysregulated expression regardless of the specific fusion gene, it allows for simultaneous morphological comparison, and it can be rapidly integrated into the routine laboratory workflow. ALK testing requires stringent internal quality controls including appropriate use of ALK-rearranged lung cancers as positive controls as well as participation in external quality assurance programs that assess performance of ALK testing. Quality assurance measures are crucial to ensure a sufficient standard of staining quality and accurate interpretation to minimize false positives and negatives, which could lead to inappropriate treatment choices and detrimentally effect patient care.35
The early studies evaluating ALK fusion immunohistochemistry reported limitations of sensitivity, describing an absence of immunoreactivity in ALK fusion positive NSCLCs.29, 36 In addition, immunohistochemistry can be hindered by poor fixation, variability in staining protocols, limited expertise by pathologists and can be hampered by small biopsies containing minimal tumor cells.27, 37 However, many subsequent studies reported that ALK immunohistochemistry is a highly sensitive method with a significant correlation with ALK FISH using a number of antibodies including the ALK1, 5A4 and D5F3 clones.15, 19, 28, 31, 34, 38, 39, 40, 41 Apart from our study, only one prior group has investigated the performance of all three antibodies in the same patient population,34 in which they found a very similar incidence of ALK rearrangement (1%) and one false-negative result using the ALK1 antibody. Many studies have reported ALK immunohistochemistry as a useful method for confirming ALK translocation by FISH.42, 43 In fact, a novel ALK fusion gene (KIF5B–ALK) was discovered following detection using an immunohistochemistry-based method.15 Differences in antibody staining intensity have also been reported,28, 34 which is validated by the data presented here, with the 5A4 and D5F3 clones showing higher staining intensity than the ALK1 clone.
One important observation of clinical importance from our study is that even low intensity immunohistochemistry staining can be associated with ALK FISH positivity, suggesting that cases with any staining intensity should be referred for confirmatory testing with ALK FISH. Unlike anaplastic large cell lymphoma, where there is typically strong nuclear and cytoplasmic expression of ALK protein associated with the commonest ALK rearrangement,6 in NSCLC the expression is more modest and cytoplasmic. In addition, our observation of heterogeneity of immunohistochemistry staining between cores of the same tumor suggests whole sections of tumors may be more representative and caution may be required when interpreting ALK immunohistochemistry in small biopsy samples where there may be a potential for false-negative immunohistochemistry.
Interestingly, one case in our data set that was technically FISH negative but with narrow signal separation was positive with all three ALK antibodies. A case report in the literature44 has also described a novel EML4–ALK rearrangement that was ALK FISH negative but immunohistochemistry positive, identified using next-generation sequencing. For the case we describe in our study, clinical response to crizotinib is not known, as this patient died some years ago. However, in light of a recent report of a patient whose tumor was positive with the 5A4 clone (Novocastra) and ALK FISH negative but showed dramatic clinical response with crizotinib,45 further research into the sensitivity of patients with ALK immunohistochemistry-positive, ALK FISH-negative tumors to crizotinib is needed. Furthermore, it has been reported that the subtle signal separation because of the chromosomal inversion producing the EML4–ALK translocation variant 1, confirmed by reverse transcription-polymerase chain reaction, is more likely to be missed by FISH.46 Wallander et al46 reported that only 1/9 (11%) of variant 1 cases were designated positive by three FISH viewers. Sensitivity and specificity was increased following a combination of scoring FISH break-apart signals that were less than two signal distances apart, and increasing the cutoff to >20% of cells with positive FISH signal patterns.
In conclusion, ALK immunohistochemistry is a valuable and cost-effective screening method that can help to enrich for cases with ALK rearrangement. However, it is important to appreciate the limitations of immunohistochemistry, which include staining heterogeneity, variable or subtle cytoplasmic expression, and the need for highly specific protocols for lung tissue. Effective routine immunohistochemistry screening depends on the use of appropriate controls, standardized protocols with careful selection of antibody and detection method, and ongoing quality assurance procedures to ensure accurate identification of ALK rearranged lung cancers. With these procedures in place, a suggested strategy could involve screening of all non-squamous lung tumors with ALK immunohistochemistry. Positive immunohistochemistry cases, or negative cases with clinicopathologic features indicative of higher pre-test likelihood of ALK rearrangement, for example, patients who have never smoked with advanced NSCLC of adenocarcinoma histology and EGFR and KRAS wild type (36% of this enriched population are reported to be ALK rearrangement positive33), or signet ring or mucinous histologic features,19, 29, 47, 48 could be evaluated using ALK FISH. The use of these integrated rigorous molecular screening methodologies may enable accurate, efficient and cost-effective identification of lung cancer patients likely to benefit from targeted anti-ALK therapies.
References
Parkin DM . Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533–543.
Jemal A, Siegel R, Ward E et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106–130.
Bell DW, Lynch TJ, Haserlat SM et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol 2005;23:8081–8092.
Gao G, Ren S, Li A et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: a meta-analysis from six phase III randomized controlled trials. Int J Cancer 2012;131:E822–E829.
Sharma SV, Bell DW, Settleman J, Haber DA . Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169–181.
Pulford K, Lamant L, Morris SW et al. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997;89:1394–1404.
Solomon B, Varella-Garcia M, Camidge DR . ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J Thorac Oncol 2009;4:1450–1454.
Rikova K, Guo A, Zeng Q et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190–1203.
Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–566.
Eudy JD, Ma-Edmonds M, Yao SF et al. Isolation of a novel human homologue of the gene coding for echinoderm microtubule-associated protein (EMAP) from the Usher syndrome type 1a locus at 14q32. Genomics 1997;43:104–106.
Pollmann M, Parwaresch R, Adam-Klages S et al. Human EML4, a novel member of the EMAP family, is essential for microtubule formation. Exp Cell Res 2006;312:3241–3251.
Chiarle R, Voena C, Ambrogio C et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 2008;8:11–23.
Choi YL, Takeuchi K, Soda M et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res 2008;68:4971–4976.
Soda M, Takada S, Takeuchi K et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci USA 2008;105:19893–19897.
Takeuchi K, Choi YL, Togashi Y et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 2009;15:3143–3149.
Togashi Y, Soda M, Sakata S et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PLoS One 2012;7:e31323.
Pao W, Girard N . New driver mutations in non-small-cell lung cancer. Lancet Oncol 2012;12:175–180.
Inamura K, Takeuchi K, Togashi Y et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13–17.
Jokoji R, Yamasaki T, Minami S et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010;63:1066–1070.
Horn L, Pao W . EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol 2009;27:4232–4235.
Koivunen JP, Mermel C, Zejnullahu K et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275–4283.
Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693–1703.
Shaw AT. . Phase III study of crizotinib versus pemetrexed or docetaxel chemotherapy in patients with advanced ALK-positive non-small cell lung cancer (NSCLC) (PROFILE 1007) [abstract]. Ann Oncol 2012;23 (Suppl 11):xi29–xi34.
Cooper WA, Kohonen-Corish MR, Chan C et al. Prognostic significance of DNA repair proteins MLH1, MSH2 and MGMT expression in non-small-cell lung cancer and precursor lesions. Histopathology 2008;52:613–622.
Selinger CI, Cooper WA, Al-Sohaily S et al. Loss of special AT-rich binding protein 1 expression is a marker of poor survival in lung cancer. J Thorac Oncol 2011;6:1179–1189.
Yip PY, Yu B, Cooper WA et al. Patterns of DNA mutations and ALK rearrangement in resected node negative lung adenocarcinoma. J Thorac Oncol 2013;8:408–414.
Camidge DR, Kono SA, Flacco A et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res 2010;16:5581–5590.
Mino-Kenudson M, Chirieac LR, Law K et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 2010;16:1561–1571.
Rodig SJ, Mino-Kenudson M, Dacic S et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216–5223.
Shaw AT, Yeap BY, Mino-Kenudson M et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247–4253.
Yi ES, Boland JM, Maleszewski JJ et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459–465.
Thunnissen E, Bubendorf L, Dietel M et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch 2012;461:245–257.
Atherly AJ, Camidge DR . The cost-effectiveness of screening lung cancer patients for targeted drug sensitivity markers. Br J Cancer 2012;106:1100–1106.
Conklin CM, Craddock KJ, Have C et al. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol 2013;8:45–51.
Francis GD, Dimech M, Giles L, Hopkins A . Frequency and reliability of oestrogen receptor, progesterone receptor and HER2 in breast carcinoma determined by immunohistochemistry in Australasia: results of the RCPA Quality Assurance Program. J Clin Pathol 2007;60:1277–1283.
Martelli MP, Sozzi G, Hernandez L et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 2009;174:661–670.
Murakami Y, Mitsudomi T, Yatabe Y . A screening method for the ALK fusion gene in NSCLC. Front Oncol 2012;2:24.
Boland JM, Erdogan S, Vasmatzis G et al. Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional up-regulation in non-small cell lung carcinomas. Hum Pathol 2009;40:1152–1158.
McLeer-Florin A, Moro-Sibilot D, Melis A et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol 2012;7:348–354.
Paik JH, Choe G, Kim H et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011;6:466–472.
Park HS, Lee JK, Kim DW et al. Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer 2012;77:288–292.
Shaw AT, Forcione DG, Digumarthy SR, Iafrate AJ. . Case records of the Massachusetts General Hospital. Case 21-2011. A 31-year-old man with ALK-positive adenocarcinoma of the lung. N Engl J Med 2011;365:158–167.
Yamamoto M, Takeuchi K, Shimoji M et al. Small non-mucinous bronchioloalveolar carcinoma with anaplastic lymphoma kinase immunoreactivity: a novel ALK fusion gene? Cancer Sci 2012;103:390–392.
Peled N, Palmer G, Hirsch FR et al. Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol 2012;7:e14–e16.
Sun JM, Choi YL, Won JK et al. A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISH-negative ALK. J Thorac Oncol 2012;7:e36–e38.
Wallander ML, Geiersbach KB, Tripp SR, Layfield LJ . Comparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated protein like 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma: implications for optimal clinical testing. Arch Pathol Lab Med 2012;136:796–803.
Popat S, Gonzalez D, Min T et al. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer 2012;75:300–305.
Yoshida A, Tsuta K, Nakamura H et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011;35:1226–1234.
Acknowledgements
We thank Thang Tran who kindly assisted with statistical analysis. This study was funded by The Sydney Foundation for Medical Research, Cancer Institute NSW, The Sydney Breast Cancer Foundation and Victorian Cancer Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Benjamin Solomon and Stephen Fox have served on advisory boards for Pfizer and Novartis. Wendy Cooper has served on advisory boards for Pfizer as well as a steering committee for an oncology forum. She has received an honorarium from Pfizer for giving a lecture at a local oncology conference. Gavin Wright has joined an advisory board for Pfizer, without remuneration.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Selinger, C., Rogers, TM., Russell, P. et al. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol 26, 1545–1553 (2013). https://doi.org/10.1038/modpathol.2013.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.87
Keywords
This article is cited by
-
The Presence of ALK Alterations and Clinical Relevance of Crizotinib Treatment in Pediatric Solid Tumors
Pathology & Oncology Research (2019)
-
Epithelioid cell histiocytoma with SQSTM1-ALK fusion: a case report
Diagnostic Pathology (2018)
-
RNA-based analysis of ALK fusions in non-small cell lung cancer cases showing IHC/FISH discordance
BMC Cancer (2018)
-
Optimized immunohistochemistry using the D5F3 antibody provides a reliable test for identification of ALK-positive lung adenocarcinomas
Virchows Archiv (2017)
-
Inconsistent results in the analysis of ALK rearrangements in non-small cell lung cancer
BMC Cancer (2016)