Abstract
Vertebrate innate immunity provides a first line of defence against pathogens such as viruses and bacteria. Viral infection activates a potent innate immune response, which can be triggered by double-stranded (ds)RNA produced during viral replication1,2,3. Here, we report that mammalian cells lacking the death-domain-containing protein FADD4,5 are defective in intracellular dsRNA-activated gene expression, including production of type I (α/β) interferons, and are thus very susceptible to viral infection. The signalling pathway incorporating FADD is largely independent of Toll-like receptor 3 and the dsRNA-dependent kinase PKR, but seems to require receptor interacting protein 1 as well as Tank-binding kinase 1-mediated activation of the transcription factor IRF-3. The requirement for FADD in mammalian host defence is evocative of innate immune signalling in Drosophila, in which a FADD-dependent pathway responds to bacterial infection by activating the transcription of antimicrobial genes6. These data therefore suggest the existence of a conserved pathogen recognition pathway in mammalian cells that is essential for the optimal induction of type I interferons and other genes important for host defence.
Similar content being viewed by others
Main
A major consequence of virus infection is the induction of the type I interferons (IFNs), which are a family of cytokines essential for host defence3,7. While investigating the mechanisms of IFN antiviral activity, we noticed that early passage murine embryonic fibroblasts (MEFs) lacking the apoptosis adaptor molecule FADD appeared to be overtly sensitive to virus infection8. To examine this phenotype further, we infected Fadd+/- and Fadd-/- MEFs with the IFN-sensitive prototypic rhabdovirus vesicular stomatitis virus (VSV), and observed that VSV replication was markedly increased (> 100-fold) in the Fadd-/- MEFs compared with their wild-type counterparts (Fig. 1a–c). Moreover, whereas pre-treatment with type I (α/β) or type II (γ) IFN for 12 h was seen to exert significant antiviral activity in normal cells, these key antiviral cytokines only delayed the onset of viral replication in Fadd-/- MEFs for 24–36 h, whereupon virus replication proceeded unchecked (Fig. 1a–c). The observed susceptibility to infection did not appear to be restricted to VSV, because MEFs lacking FADD were also sensitive to infection by other viruses, including influenza virus and encephalomyocarditis (EMCV) virus (Supplementary Fig. 1). MEFs lacking caspase 8 (refs 9, 10) (or cells treated with caspase inhibitors) exhibited no increased susceptibility to VSV infection compared to control cells, and retained the ability to be protected by IFN, suggesting that FADD exerts its antiviral effects independently of caspase 8 (Supplementary Fig. 2 and data not shown).
a, Infection of Fadd+/- and Fadd-/- MEFs with VSV (multiplicity of infection (MOI) = 10) after IFN-α/β (100 U ml-1) or IFN-γ (0.5 ng ml-1) pre-treatment. b, Viability of infected cells. c, Progeny virion production from infected cells. PFU, plaque-forming units. d, Induction of IFN-responsive genes in IFN-treated Fadd+/- and Fadd-/- MEFs. e, Protection of Fadd-/- MEFs by continuous supplementation of IFN-α/β (100 U ml-1). f, Progeny virion production from infected cells. ND, not detectable. g, Neutralizing IFN-α or IFN-β after VSV infection renders Fadd+/- cells susceptible to infection despite IFN pre-treatment. h, Progeny virion production from infected cells. Error bars are mean ± s.d.
Because exposure to type I and II IFNs was unable to effectively protect Fadd-/- MEFs from virus replication, it is plausible that effective IFN-mediated, janus kinase (JAK)-activated STAT (signal transducer and activator of transcription) signalling11 may require FADD. However, neither nuclear translocation of STAT1 nor the induction of selected type I and II IFN-responsive genes appeared to be impaired in Fadd-/- cells, indicating that IFN signalling per se is probably not compromised (Fig. 1d; see also Supplementary Fig. 3).
Despite these observations, it remained possible that the antiviral state initially established by 12 h of pre-exposure to exogenous IFN is short-lived and may require constant de novo synthesis of type I IFNs after virus infection (Fig. 1a). Indeed, we noted that continuous supplementation of recombinant type I IFNs to Fadd-/- cells after VSV infection conferred some protection against viral replication and cytolysis (Fig. 1e, f). The continual requirement for IFN induction after virus infection was further emphasized by demonstrating that antibody-mediated neutralization of secreted IFN-α or -β after exposure to VSV rendered normal cells susceptible to infection even after IFN pre-treatment (Fig. 1g, h).
These analyses suggest that an actual defect in the production of type I IFNs after virus/dsRNA detection might be responsible for the susceptibility of Fadd-/- cells to infection. To examine this possibility, we transfected Fadd+/- and Fadd-/- cells with a luciferase reporter construct under control of the IFN-β promoter and subsequently administered poly(I:C), a synthetic mimetic of viral dsRNA thought to be the primary trigger of IFN production after virus infection3. Notably, we found that transfected poly(I:C) triggered robust (∼ 10-fold) induction of the IFN-β promoter in Fadd+/- cells but not in Fadd-/- cells (Fig. 2a). The induction of the IFN-β promoter was not observed using non-transfected, exogenous poly(I:C) alone (Fig. 2a). Poly(I:C)-induced activation of IFN-β promoter-driven luciferase activity could be partially restored by reintroducing murine FADD into Fadd-/- MEFs (Supplementary Fig. 4). A requirement for FADD in dsRNA-induced activation of the IFN-β promoter was further confirmed by short interfering RNA (siRNA)-mediated suppression of FADD in HeLa cells (Supplementary Fig. 4).
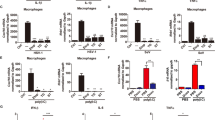
a, Defective IFN-β promoter activation by transfected poly(I:C) in Fadd-/- MEFs. LF, Lipofectamine2000. b, DNA microarray analysis of a selected set of antiviral genes. Induced levels of these genes in Fadd+/- cells were arbitrarily set to 1 (yellow) in this and other experiments. c, Relative mRNA expression levels of a selection of antiviral genes measured by real time RT–PCR. A detailed time course analysis of IFN-β gene induction in Fadd+/- versus Fadd-/- cells is also shown (top left panel). d, IFN-β production after transfection of poly(I:C), or treatment with poly(I:C) alone. e, IFN-α production after transfection with poly(I:C), treatment with poly(I:C) alone, or infection with VSV (MOI = 50). f, Normal TLR signalling in the absence of FADD. All error bars indicate mean ± s.d.
To investigate the role of FADD in dsRNA-induced gene expression, we subjected dsRNA-transfected or untreated Fadd+/- and Fadd-/- MEFs to DNA microarray analysis. Over 39,000 transcripts were examined at 0, 3, 12 and 24 h after transfection. At 3 h after transfection, we noticed that the expression of type I IFN genes, especially Ifna2, Ifna4 and Ifna5, as well as several other key IFN-inducible genes, including Irf7, were markedly reduced in Fadd-/- cells compared with controls (Fig. 2b). By 12 h after transfection, the induction of type I IFN-dependent IFN genes had increased in Fadd-/- cells, but remained significantly below the levels seen in control cells (data not shown). The impairment of type I IFN induction in the absence of FADD was confirmed at the messenger RNA level by polymerase chain reaction with reverse transcription (RT–PCR), and at the protein level by enzyme-linked immunosorbent assay (ELISA) (Fig. 2c–e). Interestingly, although induction of IFN-β-Luc was severely defective in the absence of FADD, some endogenous Ifnb transcriptional activity remained evident, possibly accounting for the eventual, albeit impaired, induction of type I IFN-dependent genes observed by DNA microarray analysis (Fig. 2a–c and data not shown). This may indicate the existence of FADD-independent dsRNA signalling events, which target elements in the endogenous IFN-β promoter independent of those found in the IFN-β-Luc construct (Fig. 2d, e). Nevertheless, given that Fig. 1g, h demonstrates a requirement for de novo type I IFN production for efficient antiviral activity, these data show that a defect in the production of type I IFNs in Fadd-/- MEFs may explain their susceptibility to virus infection.
Because the induction of type I IFNs was not readily observed using non-transfected, exogenous poly(I:C) alone (Fig. 2a, c–e), we surmised that IFN production in wild-type MEFs may involve intracellular dsRNA recognition molecules such as PKR12,13,14,15. However, MEFs lacking PKR largely retained the ability to induce type I IFN and IFN-inducible genes in response to transfected dsRNA (Supplementary Fig. 5). Furthermore, it has been shown that Toll-like receptor 3 (TLR3) is involved in the recognition of extracellular dsRNA16, an event that can lead to the induction of IFN-β through the adaptor molecule TRIF/TICAM-1 (refs 17, 18). We, however, did not observe significant type I IFN induction in MEFs or HeLa cells in response to exogenous dsRNA. Indeed, our data show that siRNA-mediated depletion of FADD—but not TLR3 or PKR (or both simultaneously)—in HeLa cells results in the reduction of IFN-β promoter activity in response to transfected poly(I:C) (Supplementary Figs 4 and 5). These results are in agreement with previous findings demonstrating effective type I IFN induction by intracellular dsRNA in PKR- or TLR3-deficient cells19,20,21,22, thus suggesting the existence of alternative TLR3/PKR-independent dsRNA signalling pathways in mammalian cells.
To determine whether FADD has a downstream role in TLR signalling, we co-transfected Fadd+/- or Fadd-/- MEFs with an IFN-β-luciferase reporter construct and plasmids encoding various components of this signalling pathway. However, we observed no abrogation of TLR3-mediated induction of the IFN-β promoter in Fadd-/- cells (Fig. 2f). These results were verified by demonstrating that TRAF6- or TRIF/TICAM-1-deficient MEFs, unlike Fadd-/- MEFs, retain the ability to resist viral infection after IFN pre-treatment (data not shown). Collectively, this would suggest that FADD is not significantly involved in TLR3-activated, TRIF/TICAM-1- or TRAF6-dependent signalling.
Intriguingly, FADD has recently been reported to be involved in the innate immune response to bacterial infection in Drosophila23,24. In this organism, the imd gene product, a homologue of the mammalian DD-containing kinase RIP1, associates with Drosophila (d)FADD to trigger activation of an NF-κB-related pathway and the subsequent induction of antibacterial genes6. To determine whether an IMD-like pathway involving FADD exists in mammalian cells, we infected IFN-treated or untreated early-passage wild-type (Ripk1+/+) and RIP1-deficient (Ripk1-/-) MEFs25 with VSV. We found that VSV induced cytolysis in Ripk1-/- cells even in the presence of IFN, similar to Fadd-/- MEFs (Fig. 3a, b). Approximately 10- to 50-fold more VSV was generated in IFN-treated Ripk1-/- MEFs compared with wild-type MEFs (Supplementary Fig. 6). Although IFN-mediated JAK/STAT signalling was found to be intact, Ripk1-/- MEFs, as well as HeLa cells in which RIP1 expression was abrogated using RNA interference, exhibited a selective and evident inability to respond to intracellular dsRNA-mediated activation of the IFN-β promoter (Fig. 3c, d; see also Supplementary Fig. 6). Overexpression of TLR3, IRAK1 and TRAF6 retained the ability to stimulate the IFN-β promoter, suggesting that RIP1 is probably not involved in the TLR3 arm of IFN-β induction, in agreement with other studies26 (Supplementary Fig. 6). DNA microarray analysis also confirmed that Ripk1-/- cells exhibit a defect in type I IFN production after exposure to intracellular dsRNA (Fig. 3e). Notably, a lack of type I IFN induction was confirmed at the protein level by IFN-β and IFN-α ELISA (Fig. 3f, g). Thus, RIP1 seems to be required for efficient intracellular, dsRNA-mediated induction of type I IFNs.
a, Infection of Ripk1+/+ and Ripk1-/- MEFs with VSV (MOI = 10) after pre-treatment with IFN-α/β (100 U ml-1) or IFN-γ (0.5 ng ml-1). b, Viability of infected cells. c, Defective IFN-β promoter activation by transfected poly(I:C) in Ripk1-/- MEFs. d, Defective IFN-β promoter activation by transfected poly(I:C) (2 µg ml-1) in RIP1 siRNA-treated HeLa cells. e, DNA microarray analysis of a selected set of antiviral genes. f, IFN-β production after transfection with poly(I:C), or treatment with poly(I:C) alone. g, IFN-α production after transfection with poly(I:C), or treatment with poly(I:C) alone. Error bars indicate mean ± s.d.
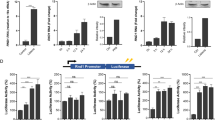
In Drosophila, IMD and dFADD are required to stimulate the induction of antimicrobial gene expression through activation of the NF-κB homologue Relish via an I-κB kinase (IKK) complex comprised of IKK-β/IRD5 and IKK-γ/Kenny6. In mammalian cells, induction of IFN-β also involves IKK-mediated activation of NF-κB, as well as IRF-3 (ref. 27). However, pre-treatment with IFN was able to effectively protect MEFs lacking IKK-α, -β or -γ against virus infection (Fig. 4a). This study was complemented by examining MEFs lacking Tank-binding kinase 1 (TBK-1; also known as IKK-δ, NAK and T2K), as this molecule seems to be the primary IRF-3 kinase in MEFs19,28,29. This experiment revealed that, similar to Fadd-/- and Ripk1-/- fibroblasts, TBK-1-deficient cells are not protected against virus replication and cytolysis even after pre-treatment with IFN (Fig. 4a; see also Supplementary Fig. 7). Similar to the situation in Fadd-/- cells, these data could be explained by a defect in type I IFN induction in TBK-1-deficient MEFs. Indeed, DNA microarray, RT–PCR and ELISA analyses confirmed a severe impairment of dsRNA-responsive induction of type I IFN, as well as other antiviral genes, in the absence of TBK-1, in agreement with other studies (Fig. 4b–d and data not shown)19,30. These data indicate that FADD may mediate its effects predominantly through TBK-1 activation of IRF-3. Accordingly, IRF-3 translocation, which occurs after phosphorylation by TBK-1 and IKK-ɛ28,29, was found to be defective in Fadd-/- cells after treatment with transfected dsRNA (Fig. 4e, f). Furthermore, Irf3-/- MEFs were not fully protected against virus infection after exposure to type I or II IFNs (Fig. 4g; see also Supplementary Fig. 7). Similarly, DNA microarray, RT–PCR, ELISA and RNA interference analyses confirmed a defect in the ability of intracellular dsRNA to induce type I IFN production in Irf3-/- MEFs (Fig. 4h–k, Supplementary Fig. 8 and data not shown).
a, Infection of wild-type or IKK-α-, IKK-β-, IKK-γ- and TBK-1--deficient MEFs with VSV (MOI = 10) with or without IFN-α/β (100 U ml-1) pre-treatment. b, DNA microarray analysis of a selected set of antiviral genes. c, IFN-β production after transfection with poly(I:C), or treatment with poly(I:C) alone. d, IFN-α production after transfection with the indicated amounts of poly(I:C), or treatment with poly(I:C) alone. e, Localization of IRF-3 after transfection of poly(I:C) for 1 h in Fadd+/- and Fadd-/- cells. f, Defective IRF-3-responsive promoter activation in Fadd-/- MEFs. g, Infection of Irf3+/+ and Irf3-/- MEFs with VSV (MOI = 10) with or without IFN-α/β (100 U ml-1) or IFN-γ (0.5 ng ml-1) pre-treatment. h, IFN-β production after transfection with poly(I:C), or treatment with poly(I:C) alone. i, IFN-α production after transfection with poly(I:C), or treatment with poly(I:C) alone. j, DNA microarray analysis for a selected set of antiviral genes. Error bars indicate mean ± s.d.
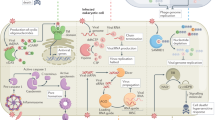
These results suggest that viral dsRNAs are recognized by an intracellular receptor molecule, which may recruit FADD and RIP1 into an ‘innateosome’ complex to regulate TBK-1-mediated activation of IRF-3. We show that the loss of FADD or RIP1 leads to a defect in IFN-β production (and perhaps the induction of other primary immune response genes) and a consequent lag in the production of IRF-7 and members of the IFN-α family, which are necessary for fortification of the antiviral state3. It is also noteworthy that TBK-1-deficient MEFs display a more profound defect in the induction of type I IFNs in response to dsRNA stimulation than either FADD-deficient or RIP1-deficient MEFs alone, plausibly suggesting that intracellular dsRNA-activated complexes retain some activity in the absence of FADD, or that alternative FADD-independent intracellular signalling cascades converge on TBK-1. This RIP1/FADD/TBK-1 (RIFT) pathway seems to be largely independent of TLR3, PKR, TRIF/TICAM-1 or TRAF6, and is in agreement with other findings suggesting the existence of alternative intracellular, dsRNA-activated signal transducers, such as the DExD/H helicase RIG-I (ref. 22) (Supplementary Fig. 9). The requirement for FADD in mammalian innate immunity is reminiscent of the IMD pathway in Drosophila, which is important for defence against Gram-negative bacteria6. Thus, similar to the Toll pathway, an alternative (FADD-dependent) innate immune pathway may be evolutionarily conserved between insects and mammals.
Methods
Cells, viruses and reagents
MEFs were provided as follows: Fadd+/-, Fadd-/-, Tbk1+/+ and Tbk1-/- (W.-C. Yeh); Casp8+/+ and Casp8-/- (D. Wallach); Traf6+/+ and Traf6-/- (J. Inoue); Ripk1+/+ and Ripk1-/- (N. Kelliher); Pkr+/+ and Pkr-/- (J. Bell and B. Williams); Stat1+/+ and Stat1-/- (J. Durbin); Trif/TICAM-1+/+ and Trif/TICAM-1-/- (S. Akira); IKK+/+, IKKα-/-, IKKβ-/- and IKKγ-/- (M. Karin); and Irf3+/+ and Irf3-/- (K. Mossman and B. Williams). All other cell lines were obtained from the American Type Culture Collection (ATCC). Poly(I:C) (Amersham-Pharmacia) was reconstituted in PBS at 2 mg ml-1, denatured at 55 °C for 30 min, and allowed to anneal to room temperature before use. Unless indicated otherwise, MEFs were transfected with 6 µg of poly(I:C) in 8 µl of Lipofectamine2000 or 100 µg poly(I:C) alone to stimulate IFN-β promoter activation and IFN production in MEFs. Luciferase assays were performed 6 h after treatment. Supernatants for ELISA were collected 24 h after treatment. VSV (Indiana strain), EMCV and influenza virus (strain A/WSN/33) were used in infections and titred by standard techniques. Human IFN-α and IFN-β and murine IFN-α ELISA Kits were acquired from PBL. Murine IFN-β ELISA assays were performed at PBL. z-VAD was from ICN Pharmaceuticals. All other reagents were from Sigma, unless mentioned otherwise.
Plasmids
Plasmids were obtained from the following sources: GFP–STAT1 (N. Reich); murine (m)FADD (ATCC); TICAM-1/TRIF, IRAK1, IRAK-M, TLR3, MyD88, TIRAP/MAL and TRAF6 (all from Invivogen); PRD I-III-Luc (T. Maniatis); IFN-β-Luc, ISRE-Luc and GAS-Luc (J. Hiscott).
RNA interference
Chemically synthesized 21-nucleotide sense and antisense RNA oligonucleotides were obtained from Dharmacon. HeLa and 293 cells were plated on six-well plates at 60,000 and 200,000 cells per well, respectively, and transfected with 100 pmol of siRNA duplex per well using Oligofectamine (Invitrogen). Assays were typically performed 72 h after siRNA treatment, when gene knockdown was found to be maximal. TLR3 and PKR siRNAs were obtained as SMARTpool proprietary sequences. Other siRNAs were as follows: hFADD, GAAGACCUGUGUGCAGCAU; mFADD, ACGAUCUGAUGGAGCUCAA; RIP1, GGCGAAGAUGAUGAACAGAUU; IRF-3, AAUCUACGAGUUUGUGAAC.
Antibodies
Antibodies were obtained from the following sources: FADD and pSTAT1 (Upstate); PKR and IRF-1 (Santa Cruz); RIP1, STAT1 and Fas (Pharmingen); β-actin (Sigma); TRAF6 (Stressgen); TBK-1/IKK-δ (Imgenex); IRF-3 (Zymed). Neutralizing antibodies to mIFN-β and mIFN-α were purchased from Research Diagnostics.
DNA microarray analysis
Total RNA was extracted from MEFs stimulated with or without poly(I:C) (6 µg ml-1 in Lipofectamine2000). Preparation of cDNA and microarray analysis was performed at the W.M. Keck Foundation Biotechnology Research Laboratory DNA microarray facility at Yale University. The mouse genome 430 2.0 array (Affymetrix) was used. Data analysis was performed with Microarray Suite software (version 5.0; Affymetrix) and GeneSpring software (Silicon Genetics).
Real-time PCR
Total RNA was isolated from cells using the RNeasy RNA extraction kit (Qiagen) and cDNA synthesis was performed using 1 µg of total RNA (Roche). Fluorescence real-time PCR analysis was performed using a LightCycler 2.0 instrument (Roche Molecular Biochemicals) and TaqMan gene expression assays (Applied Biosystems). Relative amounts of mRNA were normalized to the 18S ribosomal RNA levels in each sample.
References
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003)
Medzhitov, R. & Janeway, C. Jr. The Toll receptor family and microbial recognition. Trends Microbiol. 8, 452–456 (2000)
Taniguchi, T. & Takaoka, A. The interferon-α/β system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14, 111–116 (2002)
Chinnaiyan, A. M., O'Rourke, K., Tewari, M. & Dixit, V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505–512 (1995)
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998)
Hoffmann, J. A. The immune response of Drosophila. Nature 426, 33–38 (2003)
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998)
Balachandran, S. et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J. Virol. 74, 1513–1523 (2000)
Varfolomeev, E. E. et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998)
Muzio, M. et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85, 817–827 (1996)
Levy, D. E. & Darnell, J. E. Jr. Stats: transcriptional control and biological impact. Nature Rev. Mol. Cell Biol. 3, 651–662 (2002)
Williams, B. R. PKR; a sentinel kinase for cellular stress. Oncogene 18, 6112–6120 (1999)
Balachandran, S. et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13, 129–141 (2000)
Chu, W. M. et al. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11, 721–731 (1999)
Diebold, S. S. et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424, 324–328 (2003)
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001)
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nature Immunol. 4, 161–167 (2003)
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003)
Hemmi, H. et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199, 1641–1650 (2004)
Honda, K. et al. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl Acad. Sci. USA 100, 10872–10877 (2003)
Hoebe, K. et al. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nature Immunol. 4, 1223–1229 (2003)
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004)
Leulier, F., Vidal, S., Saigo, K., Ueda, R. & Lemaitre, B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr. Biol. 12, 996–1000 (2002)
Naitza, S. et al. The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity 17, 575–581 (2002)
Kelliher, M. A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998)
Meylan, E. et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nature Immunol. 5, 503–507 (2004)
Wathelet, M. G. et al. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1, 507–518 (1998)
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003)
Fitzgerald, K. A. et al. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nature Immunol. 4, 491–496 (2003)
McWhirter, S. M. et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl Acad. Sci. USA 101, 233–238 (2004)
Acknowledgements
We are grateful to W.-C. Yeh, N. Kelliher, D. Wallach, J. Inoue, J. Durbin, J. Bell, K. Mossman, E. Harhaj, M. Karin, B. Williams and S. Akira for fibroblasts, and T. Maniatis, J. Hiscott and N. Reich and for plasmid constructs. We also thank G. Spruill, M. Fallahi and T. Andrew for technical assistance. This work was supported by DARPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Absence of FADD sensitizes cells to infection by Encephalomyocarditis Virus (EMCV) and Influenza Virus (FLU). (JPG 82 kb)
Supplementary Figure 2
Caspase 8 is not required for protection against VSV. (JPG 96 kb)
Supplementary Figure 3
Normal IFN signalling in the absence of FADD. (JPG 23 kb)
Supplementary Figure 4
Confirmation of a requirement for FADD for optimal induction of IFN-β following intracellular dsRNA treatment. (JPG 37 kb)
Supplementary Figure 5
TLR3 and PKR independent signalling by intracellular dsRNA. (JPG 72 kb)
Supplementary Figure 6
Analysis of RIPK1 -/- MEFs. (JPG 37 kb)
Supplementary Figure 7
Quantitation of cell death and virus yield from Tbk1/Ikkδ+/+ and Tbk1/Ikkδ-/-, as well as Irf3+/+ and Irf3-/- MEFs. (JPG 51 kb)
Supplementary Figure 8
FADD, RIP1 and IRF-3 are required for IFN-α production in 293 cells. (JPG 79 kb)
Supplementary Figure 9
The RIP/FADD/TBK-1 (RIFT) cascade. (JPG 29 kb)
Supplementary Figure Legends
Legends to accompany the above figures. (DOC 55 kb)
Rights and permissions
About this article
Cite this article
Balachandran, S., Thomas, E. & Barber, G. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432, 401–405 (2004). https://doi.org/10.1038/nature03124
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03124
This article is cited by
-
Differences in IFNβ secretion upon Rab1 inactivation in cells exposed to distinct innate immune stimuli
Cellular & Molecular Immunology (2021)
-
Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study
Signal Transduction and Targeted Therapy (2020)
-
Therapeutic targeting of the PI4K2A/PKR lysosome network is critical for misfolded protein clearance and survival in cancer cells
Oncogene (2020)
-
Novel insights into the host immune response of chicken Harderian gland tissue during Newcastle disease virus infection and heat treatment
BMC Veterinary Research (2018)
-
OTULIN limits cell death and inflammation by deubiquitinating LUBAC
Nature (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.