Abstract
Pathogenic bacteria often use effector molecules to increase virulence. In most cases, the mode of action of effectors remains unknown. Strains of Pseudomonas syringae pv. syringae (Pss) secrete syringolin A (SylA), a product of a mixed non-ribosomal peptide/polyketide synthetase, in planta1. Here we identify SylA as a virulence factor because a SylA-negative mutant in Pss strain B728a obtained by gene disruption was markedly less virulent on its host, Phaseolus vulgaris (bean). We show that SylA irreversibly inhibits all three catalytic activities of eukaryotic proteasomes, thus adding proteasome inhibition to the repertoire of modes of action of virulence factors. The crystal structure of the yeast proteasome in complex with SylA revealed a novel mechanism of covalent binding to the catalytic subunits. Thus, SylA defines a new class of proteasome inhibitors that includes glidobactin A (GlbA), a structurally related compound from an unknown species of the order Burkholderiales2, for which we demonstrate a similar proteasome inhibition mechanism. As proteasome inhibitors are a promising class of anti-tumour agents, the discovery of a novel family of inhibitory natural products, which we refer to as syrbactins, may also have implications for the development of anti-cancer drugs3. Homologues of SylA and GlbA synthetase genes are found in some other pathogenic bacteria, including the human pathogen Burkholderia pseudomallei, the causative agent of melioidosis4. It is thus possible that these bacteria are capable of producing proteasome inhibitors of the syrbactin class.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Atomic coordinates have been deposited in the RCSB Protein Data Bank (http://www.rcsb.org/pdb) under the accession code 2ZCY (yeast 20S proteasome–syringolin A complex) and 3BDM (yeast 20S proteasome–glidobactin A complex).
References
Wäspi, U., Blanc, D., Winkler, T., Ruedi, P. & Dudler, R. Syringolin, a novel peptide elicitor from Pseudomonas syringae pv. syringae that induces resistance to Pyricularia oryzae in rice. Mol. Plant Microbe Interact. 11, 727–733 (1998)
Amrein, H. et al. Functional analysis of genes involved in the synthesis of syringolin A by Pseudomonas syringae pv. syringae B301D-R. Mol. Plant Microbe Interact. 17, 90–97 (2004)
Borissenko, L. & Groll, M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem. Rev. 107, 687–717 (2007)
Schellenberg, B., Bigler, L. & Dudler, R. Identification of genes involved in the biosynthesis of the cytotoxic compound glidobactin from a soil bacterium. Environ. Microbiol. 9, 1640–1650 (2007)
Hirano, S. S. & Upper, C. D. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae – a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64, 624–653 (2000)
Michel, K., Abderhalden, O., Bruggmann, R. & Dudler, R. Transcriptional changes in powdery mildew infected wheat and Arabidopsis leaves undergoing syringolin-triggered hypersensitive cell death at infection sites. Plant Mol. Biol. 62, 561–578 (2006)
Fleming, J. A. et al. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc. Natl Acad. Sci. USA 99, 1461–1466 (2002)
Meiners, S. et al. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of mammalian proteasomes. J. Biol. Chem. 278, 21517–21525 (2003)
Colon-Carmona, A., You, R., Haimovitch-Gal, T. & Doerner, P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 20, 503–508 (1999)
Doerner, P., Jorgensen, J. E., You, R., Steppuhn, J. & Lamb, C. Control of root growth and development by cyclin expression. Nature 380, 520–523 (1996)
King, R. W., Deshaies, R. J., Peters, J. M. & Kirschner, M. W. How proteolysis drives the cell cycle. Science 274, 1652–1659 (1996)
Brukhin, V., Gheyselinck, J., Gagliardini, V., Genschik, P. & Grossniklaus, U. The RPN1 subunit of the 26S proteasome in Arabidopsis is essential for embryogenesis. Plant Cell 17, 2723–2737 (2005)
Meng, L. H. et al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl Acad. Sci. USA 96, 10403–10408 (1999)
Groll, M. et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 386, 463–471 (1997)
Brünger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Oka, M. et al. Glidobactins A, B and C, new antitumor antibiotics. I. Production, isolation, chemical properties and biological activity. J. Antibiot. (Tokyo) 41, 1331–1337 (1988)
Desveaux, D., Singer, A. U. & Dangl, J. L. Type III effector proteins: doppelgangers of bacterial virulence. Curr. Opin. Plant Biol. 9, 376–382 (2006)
Galán, J. E. & Wolf-Watz, H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573 (2006)
Grant, S. R., Fisher, E. J., Chang, J. H., Mole, B. M. & Dangl, J. L. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60, 425–449 (2006)
Coleman, C. S. et al. Syringolin A, a new plant elicitor from the phytopathogenic bacterium Pseudomonas syringae pv. syringae, inhibits the proliferation of neuroblastoma and ovarian cancer cells and induces apoptosis. Cell Prolif. 39, 599–609 (2006)
Nomura, K. et al. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 313, 220–223 (2006)
Rosebrock, T. R. et al. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448, 370–374 (2007)
Dong, W. B., Nowara, D. & Schweizer, P. Protein polyubiquitination plays a role in basal host resistance of barley. Plant Cell 18, 3321–3331 (2006)
Goritschnig, S., Zhang, Y. L. & Li, X. The ubiquitin pathway is required for innate immunity in Arabidopsis. Plant J. 49, 540–551 (2007)
Adams, J. Proteasome inhibitors as new anticancer drugs. Curr. Opin. Oncol. 14, 628–634 (2002)
Quinones, B., Dulla, G. & Lindow, S. E. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant Microbe Interact. 18, 682–693 (2005)
Wäspi, U., Schweizer, P. & Dudler, R. Syringolin reprograms wheat to undergo hypersensitive cell death in a compatible interaction with powdery mildew. Plant Cell 13, 153–161 (2001)
Fenteany, G. et al. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268, 726–731 (1995)
Wallick, C. J. et al. Key role for p27(Kip1), retinoblastoma protein Rb, and MYCN in polyamine inhibitor-induced G(1) cell cycle arrest in MYCN-amplified human neuroblastoma cells. Oncogene 24, 5606–5618 (2005)
Geerts, D. et al. Expression of PRA1 domain family, member 2 (PRAF2) in neuroblastoma: correlation with clinical features, cellular localization, and cerulenin-mediated apoptosis regulation. Clin. Cancer Res. 13, 6312–6319 (2007)
Heiligtag, S. J., Bredehorst, R. & David, K. A. Key role of mitochondria in cerulenin-mediated apoptosis. Cell Death Differ. 9, 1017–1025 (2002)
Rodrigues-Pousada, R. A. et al. The Arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene-1 is expressed during early development. Plant Cell 5, 897–911 (1993)
Groll, M. & Huber, R. Purification, crystallization and X-ray analysis of the yeast 20S proteasomes. Methods Enzymol. 398, 329–336 (2005)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Potterton, E., Briggs, P., Turkenburg, M. & Dodson, E. A graphical user interface to the CCP4 program suite. Acta Crystallogr. D 59, 1131–1137 (2003)
Turk, D. Improvement of a Programme for Molecular Graphics and Manipulation of Electron Densities and Its Application for Protein Structure Determination. PhD thesis, Technische Univ. München. (1992)
Acknowledgements
We thank U. Grossniklaus and J. Celenza (Boston University) for the CycB1;1-GUS line. U. Grossniklaus and H. Gehring are thanked for reading the manuscript. We also thank D. Albert, H. Gehring, Z. Hasenkamp, R. Go, D. Koomoa, J. Molnar, A. Niewienda and C. Wallick for technical advice and assistance. We are grateful to L. Eberl for use of equipment. C.R.A was supported by a graduate student research assistantship from the Cell and Molecular Biology Graduate Program, University of Hawaii. Support by grants from the Swiss National Science Foundation to R.D. is acknowledged.
Author Contributions M.K., M.G., S.L., R.D. and A.S.B. designed experiments, analysed results and wrote the manuscript. R.D. constructed the sylA-negative mutant, T.K.P. performed the bean inoculation experiments, B.S. isolated SylA and GlbA, and performed in vitro proteasome and protease inhibition assays. M.G., M.K and R.H. performed and analysed crystallization experiments. C.R.A. performed the mammalian cell-based proteasome inhibition assays and immunoblot studies.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
The file contains Supplementary Figures 1 - 4 with Legends, Supplementary Table 1 and additional references.
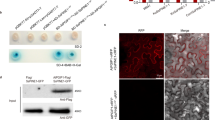
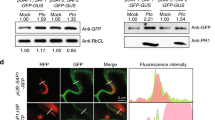
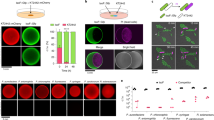
The Supplementary Figure 1 shows results confirming that SylA inhibits proteasomes irreversibly. The Supplementary Figure 2 shows that SylA inhibits bean proteasomes in crude extracts of etiolated bean seedlings. The Supplementary Figure 3 exhibits root tips of Arabidopsis seedlings homozygous for a CyclinB1;1-uidA (GUS) reporter fusion gene stained for GUS activity, showing that SylA treatment leads to enhanced staining of dividing cells. This indicates that the fusion gene product, which is expressed only in the G2/M transition of the cell cycle and then is degraded by the proteasome, accumulates upon SylA treatment. The Supplementary Figure 4 shows the results of in vivo proteasome inhibition assays in cultured human cells using the known proteasome inhibitor epoxomicin and the apoptosis-inducing agent cerulenin as positive and negative control agents, respectively. The Supplementary Table 1 lists statistical parameters of crystallographic data collection and refinement. (PDF 484 kb)
Rights and permissions
About this article
Cite this article
Groll, M., Schellenberg, B., Bachmann, A. et al. A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature 452, 755–758 (2008). https://doi.org/10.1038/nature06782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06782
This article is cited by
-
Constitutive immune mechanisms: mediators of host defence and immune regulation
Nature Reviews Immunology (2021)
-
Synthesis of macrocyclic α-ketoamide as a selective and reversible immunoproteasome inhibitor
Medicinal Chemistry Research (2021)
-
Proteasome inhibition rapidly exacerbates photoinhibition and impedes recovery during high light stress in Chlamydomonas reinhardtii
BMC Plant Biology (2020)
-
Recent insights how combined inhibition of immuno/proteasome subunits enables therapeutic efficacy
Genes & Immunity (2020)
-
Heterologous expression and antitumor activity analysis of syringolin from Pseudomonas syringae pv. syringae B728a
Microbial Cell Factories (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.