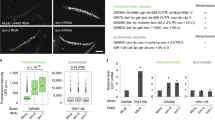
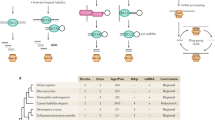
Abstract
Eukaryotic transcriptomes are characterized by widespread transcription of noncoding and antisense RNAs1,2,3, which is linked to key chromosomal processes, such as chromatin remodelling, gene regulation and heterochromatin assembly4,5,6,7. However, these transcripts can be deleterious, and their accumulation is suppressed by several mechanisms including degradation by the nuclear exosome8,9. The mechanisms by which cells differentiate coding RNAs from transcripts targeted for degradation are not clear. Here we show that the variant histone H2A.Z, which is loaded preferentially at the 5′ ends of genes by the Swr1 complex containing a JmjC domain protein, mediates suppression of antisense transcripts in the fission yeast Schizosaccharomyces pombe genome. H2A.Z is partially redundant in this regard with the Clr4 (known as SUV39H in mammals)-containing heterochromatin silencing complex that is also distributed at euchromatic loci, and with RNA interference component Argonaute (Ago1). Loss of Clr4 or Ago1 alone has little effect on antisense transcript levels, but cells lacking either of these factors and H2A.Z show markedly increased levels of antisense RNAs that are normally degraded by the exosome. These analyses suggest that as well as performing other functions, H2A.Z is a component of a genome indexing mechanism that cooperates with heterochromatin and RNAi factors to suppress read-through antisense transcripts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilhelm, B. T. et al. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature 453, 1239–1243 (2008)
Dutrow, N. et al. Dynamic transcriptome of Schizosaccharomyces pombe shown by RNA-DNA hybrid mapping. Nature Genet. 40, 977–986 (2008)
Cawley, S. et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116, 499–509 (2004)
Martens, J. A., Laprade, L. & Winston, F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429, 571–574 (2004)
Hirota, K. et al. Stepwise chromatin remodelling by a cascade of transcription initiation of non-coding RNAs. Nature 456, 130–134 (2008)
Camblong, J., Iglesias, N., Fickentscher, C., Dieppois, G. & Stutz, F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae . Cell 131, 706–717 (2007)
Grewal, S. I. & Jia, S. Heterochromatin revisited. Nature Rev. Genet. 8, 35–46 (2007)
Houseley, J., LaCava, J. & Tollervey, D. RNA-quality control by the exosome. Nature Rev. Mol. Cell Biol. 7, 529–539 (2006)
Wyers, F. et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737 (2005)
Guillemette, B. & Gaudreau, L. Reuniting the contrasting functions of H2A.Z. Biochem. Cell Biol. 84, 528–535 (2006)
Swaminathan, J., Baxter, E. M. & Corces, V. G. The role of histone H2Av variant replacement and histone H4 acetylation in the establishment of Drosophila heterochromatin. Genes Dev. 19, 65–76 (2005)
Rangasamy, D., Greaves, I. & Tremethick, D. J. RNA interference demonstrates a novel role for H2A.Z in chromosome segregation. Nature Struct. Mol. Biol. 11, 650–655 (2004)
Carr, A. M. et al. Analysis of a histone H2A variant from fission yeast: evidence for a role in chromosome stability. Mol. Gen. Genet. 245, 628–635 (1994)
Shevchenko, A. et al. Chromatin Central: towards the comparative proteome by accurate mapping of the yeast proteomic environment. Genome Biol. 9, R167 (2008)
Cam, H. P. et al. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nature Genet. 37, 809–819 (2005)
Raisner, R. M. et al. Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123, 233–248 (2005)
Zhang, H., Roberts, D. N. & Cairns, B. R. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123, 219–231 (2005)
Li, B. et al. Preferential occupancy of histone variant H2AZ at inactive promoters influences local histone modifications and chromatin remodeling. Proc. Natl Acad. Sci. USA 102, 18385–18390 (2005)
Whittle, C. M. et al. The genomic distribution and function of histone variant HTZ-1 during C. elegans embryogenesis. PLoS Genet. 4, e1000187 (2008)
Mavrich, T. N. et al. Nucleosome organization in the Drosophila genome. Nature 453, 358–362 (2008)
Zilberman, D., Coleman-Derr, D., Ballinger, T. & Henikoff, S. Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456, 125–129 (2008)
Nicolas, E. et al. Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nature Struct. Mol. Biol. 14, 372–380 (2007)
Gullerova, M. & Proudfoot, N. J. Cohesin complex promotes transcriptional termination between convergent genes in S. pombe . Cell 132, 983–995 (2008)
Zhang, K., Mosch, K., Fischle, W. & Grewal, S. I. Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nature Struct. Mol. Biol. 15, 381–388 (2008)
Murakami, H. et al. Ribonuclease activity of Dis3 is required for mitotic progression and provides a possible link between heterochromatin and kinetochore function. PLoS One 2, e317 (2007)
Li, B., Carey, M. & Workman, J. L. The role of chromatin during transcription. Cell 128, 707–719 (2007)
Roguev, A. et al. Conservation and rewiring of functional modules revealed by an epistasis map in fission yeast. Science 322, 405–410 (2008)
Andrulis, E. D. et al. The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila . Nature 420, 837–841 (2002)
Wagner, E. J. et al. A genome-wide RNA interference screen reveals that variant histones are necessary for replication-dependent histone pre-mRNA processing. Mol. Cell 28, 692–699 (2007)
Orban, T. I. & Izaurralde, E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA 11, 459–469 (2005)
Acknowledgements
We thank K. Noma for strain constructions, D. McPheeters for the run-on protocol, M. Lichten, F. Reyes-Turcu, E. Bartlett and H. Cam for comments on the manuscript, P. Fitzgerald for designing microarrays, and D. Venzon for advice with data analyses. This research was supported by the Intramural Research Program, and under Contract N01-CO-12400 of the National Institutes of Health, National Cancer Institute.
Author Contributions M.Zo., T.F. and S.I.S.G. designed research, M.Zo., T.F., K.Z. and M.Zh. performed experiments, B.C. contributed reagents, M.Zo., T.F., T.D.V. and S.I.S.G. analysed data, and S.I.S.G. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9 with Legends. (PDF 1813 kb)
Rights and permissions
About this article
Cite this article
Zofall, M., Fischer, T., Zhang, K. et al. Histone H2A.Z cooperates with RNAi and heterochromatin factors to suppress antisense RNAs. Nature 461, 419–422 (2009). https://doi.org/10.1038/nature08321
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08321
This article is cited by
-
H2A.Z marks antisense promoters and has positive effects on antisense transcript levels in budding yeast
BMC Genomics (2015)
-
The fission yeast MTREC complex targets CUTs and unspliced pre-mRNAs to the nuclear exosome
Nature Communications (2015)
-
Highly condensed chromatins are formed adjacent to subtelomeric and decondensed silent chromatin in fission yeast
Nature Communications (2015)
-
Histone exchange, chromatin structure and the regulation of transcription
Nature Reviews Molecular Cell Biology (2015)
-
Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe
Nature (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.