Abstract
Na+/Cl–-coupled biogenic amine transporters are the primary targets of therapeutic and abused drugs, ranging from antidepressants to the psychostimulants cocaine and amphetamines, and to their cognate substrates. Here we determine X-ray crystal structures of the Drosophila melanogaster dopamine transporter (dDAT) bound to its substrate dopamine, a substrate analogue 3,4-dichlorophenethylamine, the psychostimulants d-amphetamine and methamphetamine, or to cocaine and cocaine analogues. All ligands bind to the central binding site, located approximately halfway across the membrane bilayer, in close proximity to bound sodium and chloride ions. The central binding site recognizes three chemically distinct classes of ligands via conformational changes that accommodate varying sizes and shapes, thus illustrating molecular principles that distinguish substrates from inhibitors in biogenic amine transporters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carlsson, A., Lindqvist, M. & Magnusson, T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature 180, 1200 (1957)
Twarog, B. M. & Page, I. H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am. J. Physiol. 175, 157–161 (1953)
von Euler, U. Sympathin in adrenergic nerve fibres. J. Physiol. (Lond.) 105, 26 (1946)
Hertting, G. & Axelrod, J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature 192, 172–173 (1961)
Iversen, L. L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br. J. Pharmacol. 41, 571–591 (1971)
Iversen, L. L. & Kravitz, E. A. Sodium dependence of transmitter uptake at adrenergic nerve terminals. Mol. Pharmacol. 2, 360–362 (1966)
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011)
Pramod, A. B., Foster, J., Carvelli, L. & Henry, L. K. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol. Aspects Med. 34, 197–219 (2013)
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013)
Koldsø, H., Christiansen, A. B., Sinning, S. & Schiøtt, B. Comparative modeling of the human monoamine transporters: similarities in substrate binding. ACS Chem Neurosci 4, 295–309 (2013)
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl–-dependent neurotransmitter transporters. Nature 437, 215–223 (2005)
Beuming, T., Shi, L., Javitch, J. A. & Weinstein, H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol. Pharmacol. 70, 1630–1642 (2006)
Gu, H., Wall, S. C. & Rudnick, G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J. Biol. Chem. 269, 7124–7130 (1994)
Kilty, J. E., Lorang, D. & Amara, S. G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science 254, 578–579 (1991)
Kurian, M. A. et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J. Clin. Invest. 119, 1595–1603 (2009)
Giros, B., Jaber, M., Jones, S. R., Wightman, R. M. & Caron, M. G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612 (1996)
Mergy, M. A. et al. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc. Natl Acad. Sci. USA 111, E4779–E4788 (2014)
Zaczek, R., Culp, S., Goldberg, H., McCann, D. J. & De Souza, E. B. Interactions of [3H]amphetamine with rat brain synaptosomes. I. Saturable sequestration. J. Pharmacol. Exp. Ther. 257, 820–829 (1991)
Bönisch, H. The transport of (+)-amphetamine by the neuronal noradrenaline carrier. Naunyn Schmiedebergs Arch. Pharmacol. 327, 267–272 (1984)
Eshleman, A. J., Henningsen, R. A., Neve, K. A. & Janowsky, A. Release of dopamine via the human transporter. Mol. Pharmacol. 45, 312–316 (1994)
Goodwin, J. S. et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 284, 2978–2989 (2009)
Sitte, H. H. et al. Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J. Neurochem. 71, 1289–1297 (1998)
Sonders, M. S., Zhu, S. J., Zahniser, N. R., Kavanaugh, M. P. & Amara, S. G. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J. Neurosci. 17, 960–974 (1997)
Wall, S. C., Gu, H. & Rudnick, G. Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol. Pharmacol. 47, 544–550 (1995)
Beuming, T. et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nature Neurosci. 11, 780–789 (2008)
Bisgaard, H. et al. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology 60, 182–190 (2011)
Sørensen, G. et al. Neuropeptide Y Y5 receptor antagonism attenuates cocaine-induced effects in mice. Psychopharmacology (Berl.) 222, 565–577 (2012)
Richelson, E. & Pfenning, M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur. J. Pharmacol. 104, 277–286 (1984)
Sørensen, L. et al. Interaction of antidepressants with the serotonin and norepinephrine transporters: mutational studies of the S1 substrate binding pocket. J. Biol. Chem. 287, 43694–43707 (2012)
Dahal, R. A. et al. Computational and biochemical docking of the irreversible cocaine analog RTI 82 directly demonstrates ligand positioning in the dopamine transporter central substrate binding site. J. Biol. Chem. 289, 29712–29727 (2014)
Pörzgen, P., Park, S. K., Hirsh, J., Sonders, M. S. & Amara, S. G. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol. Pharmacol. 59, 83–95 (2001)
Abdul-Hussein, S., Andrell, J. & Tate, C. G. Thermostabilisation of the serotonin transporter in a cocaine-bound conformation. J. Mol. Biol. 425, 2198–2207 (2013)
Wang, H. et al. Structural basis for action by diverse antidepressants on biogenic amine transporters. Nature 503, 141–145 (2013)
Huang, X. & Zhan, C. G. How dopamine transporter interacts with dopamine: insights from molecular modeling and simulation. Biophys. J. 93, 3627–3639 (2007)
Kitayama, S. et al. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc. Natl Acad. Sci. USA 89, 7782–7785 (1992)
Han, D. D. & Gu, H. H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 6, 6 (2006)
Newman, A. H., Zou, M. F., Ferrer, J. V. & Javitch, J. A. [3H]MFZ 2–12: a novel radioligand for the dopamine transporter. Bioorg. Med. Chem. Lett. 11, 1659–1661 (2001)
Kazmier, K. et al. Conformational dynamics of ligand-dependent alternating access in LeuT. Nature Struct. Mol. Biol. 21, 472–479 (2014)
Krishnamurthy, H. & Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 481, 469–474 (2012)
Hong, W. C. & Amara, S. G. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J. Biol. Chem. 285, 32616–32626 (2010)
Zhao, Y. et al. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature 474, 109–113 (2011)
Malinauskaite, L. et al. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. Nature Struct. Mol. Biol. 21, 1006–1012 (2014)
Perez, C., Koshy, C., Yildiz, O. & Ziegler, C. Alternating-access mechanism in conformationally asymmetric trimers of the betaine transporter BetP. Nature 490, 126–130 (2012)
Weyand, S. et al. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science 322, 709–713 (2008)
Loland, C. J. et al. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol. Pharmacol. 73, 813–823 (2008)
Lee, S. H. et al. Importance of valine at position 152 for the substrate transport and 2β-carbomethoxy-3β-(4-fluorophenyl)tropane binding of dopamine transporter. Mol. Pharmacol. 57, 883–889 (2000)
Chen, J. G., Sachpatzidis, A. & Rudnick, G. The third transmembrane domain of the serotonin transporter contains residues associated with substrate and cocaine binding. J. Biol. Chem. 272, 28321–28327 (1997)
Singh, S. K., Piscitelli, C. L., Yamashita, A. & Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 322, 1655–1661 (2008)
Claxton, D. P. et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nature Struct. Mol. Biol. 17, 822–829 (2010)
Schmitt, K. C., Rothman, R. B. & Reith, M. E. Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates. J. Pharmacol. Exp. Ther. 346, 2–10 (2013)
Reeves, P. J., Callewaert, N., Contreras, R. & Khorana, H. G. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl Acad. Sci. USA 99, 13419–13424 (2002)
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nature Protocols 9, 2574–2585 (2014)
Otwinowski, Z. &. Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Quick, M. & Javitch, J. A. Monitoring the function of membrane transport proteins in detergent-solubilized form. Proc. Natl Acad. Sci. USA 104, 3603–3608 (2007)
Eshleman, A. J. et al. Metabolism of catecholamines by catechol-O-methyltransferase in cells expressing recombinant catecholamine transporters. J. Neurochem. 69, 1459–1466 (1997)
Dehnes, Y. et al. Conformational changes in dopamine transporter intracellular regions upon cocaine binding and dopamine translocation. Neurochem. Int. 73, 4–15 (2014)
Acknowledgements
We thank J. Coleman, C.-H. Lee, S. Mansoor and other Gouaux laboratory members for helpful discussions, L. Vaskalis for assistance with figures and H. Owen for help with manuscript preparation. We acknowledge the staff of the Berkeley Center for Structural Biology at the Advanced Light Source and the Northeastern Collaborative Access Team at the Advanced Photon Source for assistance with data collection. This work was supported by an NIMH Ruth Kirschstein postdoctoral fellowship and Brain and Behavior Research Foundation Young Investigator research award (K.H.W.), a postdoctoral fellowship from the American Heart Association (A.P.) and by the NIH (E.G) and the Methamphetamine Abuse Research Center of OHSU (P50DA018165 to E.G). E.G. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
A.P., K.H.W. and E.G. designed the project. A.P. and K.H.W. performed protein purification, crystallography and biochemical assays. A.P., K.H.W. and E.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Design of the minimal functional construct.
a, Thermostabilizing (ts) mutations V275A, V311A, G538L were removed. Modification of the EL2 deletion from 164–206 to 162–202, which recovered transport activity. The del 162–201 construct has robust dopamine uptake activity. b, Structural organization of EL2 regions. Organization of dDATcryst with a deletion of region 164–206 depicted as green surface. c, EL2 structure in dDATmfc with the deletion 162–202 depicted as cyan surface showing contacts between EL2 and EL6. d, EL2 organization in the construct with a deletion from 162–201 depicted as magenta surface. e, Fab 9D5 interferes with the interaction between EL2 and EL6 in the crystal lattice, with loops depicted as magenta and cyan surfaces, respectively. Fab disrupts the EL organization in all structures. The del 162–201 subB structure is shown.
Extended Data Figure 2 Measurement of dissociation constants using purified dDATmfc protein, and dopamine uptake in whole cells.
a, dDATmfc binds [3H]nisoxetine with a Kd of 36 ± 3 nM (s.e.m.). b, dDATmfc with subB mutations binds [3H]nisoxetine with a Kd of 10 ± 1 nM (s.e.m.). c, d, Michaelis–Menten plots of [14C]dopamine uptake by HEK293S cells expressing dDATwt or dDATmfc, respectively, which yielded a KM of 2.1 ± 0.7 μM and Vmax of 4.5 ± 0.4 pmol min−1 per 106 cells for dDATwt and a KM of 8.2 ± 2.3 μM and Vmax of 2.4 ± 0.2 pmol min−1 per 106 cells for dDATmfc (s.e.m.). One representative plot of total and background counts (in the presence of 10 μM nortriptyline) is shown of two experimental trials as squares and triangles, respectively. Data points and error bars show the average and standard deviation, respectively, of technical replicates (n = 3). Welch’s t-test indicates that the specific uptake signal at each concentration of dopamine is significant with a two-tailed P value < 0.02. e, Eadie–Hofstee plot of specific dopamine uptake shown in Fig. 1a and panels c and d of this figure. Data for dDATwt and dDATmfc are shown as squares and triangles, respectively, and error bars denote s.d. of technical replicates (n = 3). f, The thermal melting curve of dDATmfc solubilized from HEK293S membranes in the presence of 100 nM [3H]nisoxetine exhibits a melting temperature of 48 ± 2 °C (s.e.m.). The fraction bound describes the signal remaining after incubation at the specified temperature for 10 min, normalized to the signal at 4 °C. Data points show the mean values for one experimental trial, and error bars show the s.d. of technical replicates (n = 3).
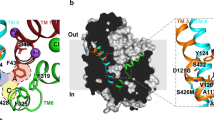
Extended Data Figure 3 Mutagenesis and effects of DAT subsite B.
a, Sequence alignment of subsite B regions for dDAT and human NSS orthologues. b, 2Fo − Fc density contoured at 0.9σ around the vicinity of the D121G (TM3) and S426M mutations (TM8). c, Abrogation of dopamine transport activity by dDATwt bearing both subsite B mutations in infected HEK293S cells. Data show the average uptake and error bars show the data range of technical duplicates for a single trial. Reactions were performed without and with 100 μM desipramine in black and grey bars, respectively.
Extended Data Figure 4 Measurement of inhibition constants using purified dDAT protein.
a–c, Inhibition of [3H]nisoxetine binding to dDATmfc (squares) and dDATmfc subB (triangles). Ki inhibition constants for dDATmfc and dDATmfc subB are, respectively, 98 ± 4 μM, and 1.4 ± 0.1 μM, (a, β-CFT), 371 ± 25 nM and 271 ± 59 nM (b, RTI-55), and 4.5 ± 0.3 μM, and 267 ± 20 nM (c, DCP). All errors are s.e.m. One representative trial of two is shown for all experiments in panels a–c, and data points and error bars denote the average values for fraction bound and standard deviation, respectively, for technical replicates (n = 3). d, Inhibition of [3H]nisoxetine (50 nM) binding to dDATdel by 1 and 10 μM unlabelled compound (grey and black bars, respectively). Error bars show the data range of technical replicates (n = 2). Abbreviations: 3-BrPE, 3-bromophenethylamine; 4-BrPE, 4-bromophenethylamine; 2-pTE, 2-(pTolyl)ethylamine, 4-ClPE, 4-chlorophenethylamine; DCP, 3,4-dichlorophenethylamine.
Extended Data Figure 5 Fo − Fc densities for ligands complexed with dDAT.
a, d-amphetamine (2.4σ); b, (+)-methamphetamine (1.8σ); c, DCP (2.2σ); d, cocaine (2.2σ); e, β-CFT (2.2σ); f, RTI-55 (2.6σ).
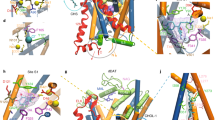
Extended Data Figure 6 Helical movements in dDATmfc upon binding to substrate analogue DCP (orange) and inhibitor nortriptyline (grey).
a–d, Helices undergoing maximal shifts are a, TM1b; b, TM2; c, TM6a; d, TM11. Arrows in black represent direction of shift. e, Table comparing angular shifts between nortriptyline–dDATcryst (PDB ID 4M48) and DCP–dDATmfc structures in column one, and between the outward-open Trp–LeuT (PDB ID 3F3A) and outward-occluded Leu–LeuT (PDB ID 2A65) structures in column two. f, Superposition of the outward open state of nortriptyline–dDATcryst (PDB ID 4M48) and DCP–dDATmfc structures in grey and orange ribbon, respectively. Extracellular gating TMs 1b and 6a are shown as cylinders. Arrows in red indicate inward movement of TMs 1b and 6a. g, Superposition of the occluded state of LeuT (PDB ID 2A65) and DCP–dDATmfc structures in grey and orange ribbon, respectively.
Extended Data Figure 7 Cholesterol binding sites in DAT.
a, Cholesterol binding sites seen on the dDAT surface corresponding to the inner leaflet of the plasma membrane, with a second novel cholesterol site into which a cholesteryl hemisuccinate (CHS) could be modelled. Fo − Fc densities for cholesterol contoured at 2.0σ. b, Close-up view of cholesterol site II at the junction of TM2, TM7 and TM11 interacting with multiple hydrophobic residues. Asterisk denotes thermostabilizing mutant V74A. c, Effect of CHS concentration on [3H]nisoxetine binding to DATmfc construct. Graph depicts one representative trial of two independent experiments, and total and background counts were measured using technical replicates (n = 3) for each binding curve at each CHS concentration. Arrow represents increasing concentration of CHS. Error bars represent s.d.
Extended Data Figure 8 Analogues of cocaine and binding site comparisons.
a, The position of RTI-55 in the binding pocket with anomalous difference density for iodide displayed as purple mesh and contoured at 4σ. b, Superposition of cocaine, β-CFT, and RTI-55 using the RTI-55-dDATmfc structure. Ligands are shown as sticks and coloured yellow (cocaine), pink (β-CFT), and teal (RTI-55). Sodium ions are shown as purple spheres. c–f, Residues that line the binding pocket are superposed between the nortriptyline–dDATcryst (magenta, PDB ID 4M48) and those of c, DA–dDATmfc (cyan), d, DCP–dDATmfc (marine), e, cocaine–dDATmfc (yellow). f, Organization of S1 binding site in complex with nortriptyline (PDB ID 4M48). Black arrows describe the change in rotamers and positions of D46, F319, and F325 compared to the nortriptyline-bound structure.
Supplementary information
Supplementary Tables
This file contains Supplementary Table 1. (PDF 209 kb)
Ligand-induced conformational rearrangements illustrate structural plasticity
Shown is a morph between selected structural states of the Drosophila dopamine transporter beginning with the previously published nortriptyline-bound outward-open state (magenta, PDB. id 4M48) followed by structural changes associated with the binding of cocaine (yellow, PDB. ids 4XP4, 4XPB) wherein Phe 325 and the TM6a-6b linker move into the binding site to interact with the benzyloxy aromatic group of cocaine. Transition from the cocaine bound state is followed by the D-amphetamine-bound (light orange, PDB. ids 4XP9) state in which additional ‘contraction’ of the TM6a-6b linker retains aromatic π-π interactions with Phe 325. The dopamine-bound conformation (cyan, PDB. id 4XP1) follows the D-amphetamine-bound state, illustrating the rotameric shift in the side chain of Asp 46, allowing it to interact with the primary amine of the neurotransmitter. Upon binding of the dopamine analogue DCP (teal, PDB. ids 4XPA, 4XPH) there are shifts in TM helices 1b (deep red) and 6a (green) and a rotameric reorientation of Phe 319 that blocks the substrate binding site from solvent access. Scaffold helices TMs 3 and 8 are colored in orange and cyan respectively. (MPG 22010 kb)
Rights and permissions
About this article
Cite this article
Wang, K., Penmatsa, A. & Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521, 322–327 (2015). https://doi.org/10.1038/nature14431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14431
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.