Abstract
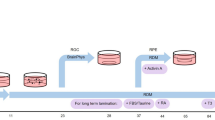
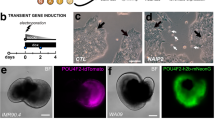
Irreversible blindness caused by loss of photoreceptors may be amenable to cell therapy. We previously demonstrated retinal repair1 and restoration of vision through transplantation of photoreceptor precursors obtained from postnatal retinas into visually impaired adult mice2,3. Considerable progress has been made in differentiating embryonic stem cells (ESCs) in vitro toward photoreceptor lineages4,5,6. However, the capability of ESC-derived photoreceptors to integrate after transplantation has not been demonstrated unequivocally. Here, to isolate photoreceptor precursors fit for transplantation, we adapted a recently reported three-dimensional (3D) differentiation protocol that generates neuroretina from mouse ESCs6. We show that rod precursors derived by this protocol and selected via a GFP reporter under the control of a Rhodopsin promoter integrate within degenerate retinas of adult mice and mature into outer segment–bearing photoreceptors. Notably, ESC-derived precursors at a developmental stage similar to postnatal days 4–8 integrate more efficiently compared with cells at other stages. This study shows conclusively that ESCs can provide a source of photoreceptors for retinal cell transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
MacLaren, R.E. et al. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203–207 (2006).
Pearson, R.A. et al. Restoration of vision after transplantation of photoreceptors. Nature 485, 99–103 (2012).
Barber, A.C. et al. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 110, 354–359 (2013).
Lamba, D.A., Karl, M.O., Ware, C.B. & Reh, T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 103, 12769–12774 (2006).
Osakada, F. et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 26, 215–224 (2008).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Bartsch, U. et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp. Eye Res. 86, 691–700 (2008).
Lakowski, J. et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum. Mol. Genet. 19, 4545–4559 (2010).
Pearson, R.A. et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 19, 487–503 (2010).
Lakowski, J. et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells 29, 1391–1404 (2011).
Eberle, D., Schubert, S., Postel, K., Corbeil, D. & Ader, M. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest. Ophthalmol. Vis. Sci. 52, 6462–6471 (2011).
Singh, M.S. et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 110, 1101–1106 (2013).
Meyer, J.S. et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 106, 16698–16703 (2009).
Hirami, Y. et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci. Lett. 458, 126–131 (2009).
Meyer, J.S. et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells 29, 1206–1218 (2011).
Phillips, M.J. et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest. Ophthalmol. Vis. Sci. 53, 2007–2019 (2012).
Osakada, F., Ikeda, H., Sasai, Y. & Takahashi, M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat. Protoc. 4, 811–824 (2009).
West, E.L. et al. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells 30, 1424–1435 (2012).
Ali, R.R. & Sowden, J.C. Regenerative medicine: DIY eye. Nature 472, 42–43 (2011).
Martinez-Morales, J.R. & Wittbrodt, J. Shaping the vertebrate eye. Curr. Opin. Genet. Dev. 19, 511–517 (2009).
Hyatt, G.A., Schmitt, E.A., Fadool, J.M. & Dowling, J.E. Retinoic acid alters photoreceptor development in vivo. Proc. Natl. Acad. Sci. USA 93, 13298–13303 (1996).
Kelley, M.W., Williams, R.C., Turner, J.K., Creech-Kraft, J.M. & Reh, T.A. Retinoic acid promotes rod photoreceptor differentiation in rat retina in vivo. Neuroreport 10, 2389–2394 (1999).
Lombardini, J.B. Taurine: retinal function. Brain Res. Brain Res. Rev. 16, 151–169 (1991).
Chen, S. et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19, 1017–1030 (1997).
Furukawa, T., Morrow, E.M. & Cepko, C.L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 91, 531–541 (1997).
Blackshaw, S. et al. Genomic analysis of mouse retinal development. PLoS Biol. 2, e247 (2004).
Calvert, P.D. et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc. Natl. Acad. Sci. USA 97, 13913–13918 (2000).
Lamba, D.A., Gust, J. & Reh, T.A. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73–79 (2009).
Koulen, P., Kuhn, R., Wässle, H. & Brandstätter, J.H. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc. Natl. Acad. Sci. USA 96, 9909–9914 (1999).
Koulen, P. & Brandstätter, J.H. Pre- and postsynaptic sites of action of mGluR8a in the mammalian retina. Invest. Ophthalmol. Vis. Sci. 43, 1933–1940 (2002).
West, E.L. et al. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp. Eye Res. 86, 601–611 (2008).
Tucker, B.A. et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE 6, e18992 (2011).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Evans, M.J. & Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Gao, G.-P. et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol. Ther. 5, 644–649 (2002).
Davidoff, A.M. et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Methods 121, 209–215 (2004).
Luhmann, U.F.O. et al. Differential modulation of retinal degeneration by Ccl2 and Cx3cr1 chemokine signalling. PLoS ONE 7, e35551 (2012).
Tschernutter, M. et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 12, 694–701 (2005).
Acknowledgements
This work was supported by the Medical Research Council UK (mr/j004553/1, G0901550), RP Fighting Blindness (GR566), The Miller's Trust and Moorfields Eye Charity through a generous private donation. A.G.-C. is a Wellcome Trust PhD student (087256/Z08/Z). R.A.P. is a Royal Society University Research Fellow. J.C.S. is supported by Great Ormond Street Hospital Children's Charity. R.R.A. is partly funded by the Department of Health's National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital and Alcon Research Institute. We thank A. Eddaoudi, A. Rose and T. Adejumo for FACS assistance; S. Azam and S. Haria for virus purification; S. Sharma for performing the Affymetrix microarray; and P. Munro for EM assistance. The mouse EK.CCE ESC line34 (129/SvEv) was a kind gift of E. Robertson. The following mouse lines were kind gifts: Gnat1−/− was provided by J. Lem, Tufts University School of Medicine; Prph2rd2/rd2 by G. Travis, UCLA; Rho−/− by P. Humphries, Trinity College Dublin and Nrlp.GFP+/+ by A. Swaroop, University of Michigan.
Author information
Authors and Affiliations
Contributions
A.G.-C. and E.L.W. contributed equally to the concept, design, execution and analysis of all experiments and manuscript writing. R.A.P. performed subretinal transplantation and calcium imaging, and contributed to the concept and design of the experiments, funding and manuscript writing. Y.D. performed subretinal transplantations and histological processing. L.S.C., A.G. and J.L. contributed to experimental execution. C.J.C. performed IMARIS reconstruction. A.N. and S.J.I.B. provided technical assistance. M.H. performed microarray data analysis. J.W.B.B., A.J.S., J.C.S. and R.R.A. contributed to the concept and design of the experiments, funding and to manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1–2 (PDF 2735 kb)
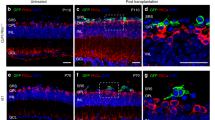
Cellular morphology and alignment of an integrated ESC-derived rod photoreceptor
Following transplantation of day 29 ESC-derived Rhop.GFP+ photoreceptors into Gnat1−/− mice, retinal flatmounts were stained for the ribbon synaptic marker, Ribeye (magenta) and rod a-Transducin (red). 3D reconstruction of a representative confocal image showing the correct position of the integrated cell in the ONL (DAPI, blue), the presence of inner and outer segments expressing rod a-Transducin, as well as an inner process ending in a spherule synaptic button correctly positioned in the OPL, where synapses were located. The 3D confocal image was reconstructed to illustrate these morphologies. The whole synaptic layer is shown first, before highlighting the synapse contacting the spherule. The surface of the entire cell was then rendered to demonstrate correct spatial alignment and morphology between the ribbon synapse in relation to the rod spherule. ONL, outer nuclear layer; OPL, outer plexiform layer. (AVI 6951 kb)
Rights and permissions
About this article
Cite this article
Gonzalez-Cordero, A., West, E., Pearson, R. et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol 31, 741–747 (2013). https://doi.org/10.1038/nbt.2643
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2643
This article is cited by
-
Automating iPSC generation to enable autologous photoreceptor cell replacement therapy
Journal of Translational Medicine (2023)
-
Machine learning-based estimation of spatial gene expression pattern during ESC-derived retinal organoid development
Scientific Reports (2023)
-
Application of Human Stem Cell Derived Retinal Organoids in the Exploration of the Mechanisms of Early Retinal Development
Stem Cell Reviews and Reports (2023)
-
Progress of iPS cell-based transplantation therapy for retinal diseases
Japanese Journal of Ophthalmology (2023)
-
Self-organization, quality control, and preclinical studies of human iPSC-derived retinal sheets for tissue-transplantation therapy
Communications Biology (2023)