Abstract
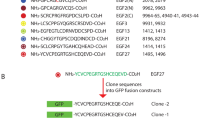
Antigenic determinants are often composed of discontiguous polypeptide segments, adjacent on protein surfaces, which are difficult to map by conventional techniques. Here we show that although monoclonal antibodies did not recognize recombinant molecules containing only the aminoterminal or only the carboxyterminal part of a protein, after in vitro refolding they are able to recognize a mixture of the two molecules. This suggests that two molecules, each containing part of a discontiguous epitope, are able to reconstitute in vitro the conformation recognized by a monoclonal antibody. Mapping of the regions required for antibody binding can therefore be achieved by in vitro refolding of recombinant molecules. Site-directed mutagenesis is then used to further define the sequences involved in antibody recognition. This new approach was used to map a discontiguous, conformational epitope on the S1 subunit of pertussis toxin. The analysis revealed that pertussis toxin is likely to contain only one immunodominant epitope, which is recognized by all seven protective monoclonal antibodies available to us. In theory, the method described can be used to map conformational epitopes in any protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tamura, M., Nogimori, L., Murai, S., Yajima, M., Itlo, K., Katada, T., Ui, M. and Ishii, S. 1982. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516–5522.
Nicosia, A., Perugini, M., Franzini, C., Casagli, C., Borri, M.G., Antoni, G., Almoni, M., Neri, P., Ratti, G. and Rappuoli, R. 1986. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc. Nail. Acad. Sci. USA 83:4631–4635.
Locht, C. and Keith, J.M. 1986. Pertussis toxin gene: nucleotide sequence and genetic organization. Science 232:11258–11264.
Sato, Y., Kimura, M. and Fukumi, H. 1984. Development of a pertussis component vaccine in Japan. The Lancet i:122–126.
Sato, H., Ito, A., Chiba, J. and Sato, Y. 1984. Monoclonal antibody against pertussis toxin: Effect on toxin activity and pertussis infections. Infect. Immun. 46:422–428.
Sato, H., Sato, Y., Ito, A. and Ohishi, I. 1987. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect. Immun. 55:909–915.
Nicosia, A., Bartoloni, A., Perugini, M. and Rappuoli, R. 1987. Expression and immunological properties of the five subunits of pertussis toxin. Infect. Immun. 55:963–967.
Burnette, W.N. 1981. “Western Blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195–203.
Zoller, M.J. and Smith, M. 1984. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA 3:479–488
Perera, V.Y., Wardlow, A.C. and Freer, J.H. 1986. Antigenic heterogeneity in subunit S1 of pertussis toxin. J. Gen. Microbiol. 132:553–556.
Anwar, H., Ashworth, L.A.E., Funnell, S., Robinson, A. and Irons, I. 1987. Neutralization of biological activities of pertussis toxin with a monoclonal antibody. FEMS Microbiology Letters 44:141–145.
Aricò, B., Gross, R., Smida, J. and Rappuoli, R. 1987. Evolutionary relationships in the genus Bordetella. Molecular Microbiology 1(3):301–308.
Amit, A.G., Mariuzza, R.A., Phillips, S.V.E. and Poljak, R.J. 1986. Three-dimensional structure of an antigen-antibody complex at 2.8 Å resolution. Science 233:747–753.
Chothia, C., Lesk, A.M., Levitt, M., Amit, A.G., Mariuzza, R.A., Phillips, E.V. and Poljak, R.J. 1986. The predicted structure of immunoglobulin D1.3 and its comparison with the crystal structure. Science 233:755–758.
Burnens, A., Demotz, S., Corradini, G., Binz, H. and Bosshard, H.R. 1987. Epitope mapping by chemical modification of free and antibody-bound protein antigen. Science 235:780–783.
Bigio, M., Rossi, R., Nucci, D., Borri, M.G., Antoni, G., Bartoloni, A. and Rappuoli, R. 1988. Monoclonal antibodies against pertussis toxin subunits. FEMS Microbiology Letters (in press).
Maniatis, T., Fritsch, E.F. and Sambrook, J. 1982. Molecular cloning, a Laboratory Manual. Cold Spring Harbor Laboratorv, Cold Spring Harbor, N.Y.
Mariani, M., Nucci, D., Bracci, L., Neri, P. and Antoni, G. 1986 Determination of antigen-specific immunoglobulin content in ascitic fluids and antisera. J. Immunol Methods 92:189–193.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bartoloni, A., Pizza, M., Bigio, M. et al. Mapping of a Protective Epitope of Pertussis Toxin by In Vitro Refolding of Recombinant Fragments. Nat Biotechnol 6, 709–712 (1988). https://doi.org/10.1038/nbt0688-709
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0688-709
This article is cited by
-
Identification of two novel rabbit hemorrhagic disease virus (RHDV) B cell epitopes and evaluation of its immunoprotection against RHDV
Applied Microbiology and Biotechnology (2015)