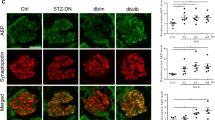
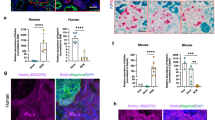
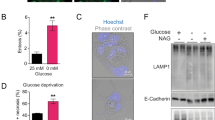
Abstract
Keratins 8 and 18 (K8 and K18) are heteropolymeric intermediate filament phosphoglycoproteins of simple-type epithelia. Mutations in K8 and K18 predispose the affected individual to liver disease as they protect hepatocytes from apoptosis. K18 undergoes dynamic O-linked N-acetylglucosamine glycosylation at Ser 30, 31 and 49. We investigated the function of K18 glycosylation by generating mice that overexpress human K18 S30/31/49A substitution mutants that cannot be glycosylated (K18–Gly−), and compared the susceptibility of these mice to injury with wild-type and other keratin-mutant mice. K18–Gly− mice are more susceptible to liver and pancreatic injury and apoptosis induced by streptozotocin or to liver injury by combined N-acetyl-D-glucosaminidase inhibition and Fas administration. The enhanced apoptosis in the livers of mice that express K18–Gly− involves the inactivation of Akt1 and protein kinase Cθ as a result of their site-specific hypophosphorylation. Akt1 binds to K8, which probably contributes to the reciprocal hyperglycosylation and hypophosphorylation of Akt1 that occurs on K18 hypoglycosylation, and leads to decreased Akt1 kinase activity. Therefore, K18 glycosylation provides a unique protective role in epithelial injury by promoting the phosphorylation and activation of cell-survival kinases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ku, N. -O., Zhou, X., Toivola, D. M. & Omary, M. B. The cytoskeleton of digestive epithelia in health and disease. Am. J. Physiol. 277, G1108–1137 (1999).
Fuchs, E. & Cleveland, D. W. A structural scaffolding of intermediate filaments in health and disease. Science 279, 514–519 (1998).
Omary, M. B., Coulombe, P. A. & McLean, W. H. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351, 2087–2100 (2004).
Coulombe, P. A. & Omary, M. B. 'Hard' and 'Soft' principles defining the strucuture, function and regulation of keratin intermediate filaments. Curr. Opin. Cell Biol. 14, 110–122 (2002).
Moll, R., Franke, W. W., Schiller, D. L., Geiger, B. & Krepler, R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982).
Ku, N. -O., Gish, R., Wright, T. L. & Omary, M. B. Keratin 8 mutations in patients with cryptogenic liver disease. N. Engl. J. Med. 344, 1580–1587 (2001).
Ku, N. -O. et al. Keratins as susceptibility genes for end-stage liver disease. Gastroenterology 129, 885–893 (2005).
Omary, M. B., Ku, N. -O., Strnad, P. & Hanada, S. Towards unraveling the complexity of 'simple' epithelial keratins in human disease. J. Clin. Invest. 119, 1794–1805 (2009).
Strnad, P. et al. Keratin variants predispose acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology 139, 828–835 (2010).
Oriolo, A. S., Wald, F. A., Ramsauer, V. P. & Salas, P. J. Intermediate filaments: a role in epithelial polarity. Exp. Cell Res. 313, 2255–2264 (2007).
Toivola, D. M., Tao, G. Z., Habtezion, A., Liao, J. & Omary, M. B. Beyond cellular integrity: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 15, 608–617 (2005).
Kim, S. & Coulombe, P. A. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat. Rev. Mol. Cell Biol. 11, 75–81 (2010).
Omary, M. B., Ku, N. -O., Tao, G. Z., Toivola, D. M. & Liao, J. 'Heads and tails' of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem. Sci. 31, 383–394 (2006).
Ku, N. -O. & Omary, M. B. A disease and phosphorylation related non-mechanical function for keratin 8. J. Cell Biol. 174, 115–125 (2006).
Chou, C. F., Smith, A. J. & Omary, M. B. Characterization and dynamics of O-linked glycosylation of human cytokeratin 8 and 18. J. Biol. Chem. 267, 3901–3906 (1992).
Ku, N. -O. & Omary, M. B. Identification and mutational analysis of the glycosylation sites of human keratin 18. J. Biol. Chem. 270, 11820–11827 (1995).
Omary, M. B., Ku, N. -O., Liao, J. & Price, D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell. Biochem. 31, 105–140 (1998).
Kudlow, J. E. Post-translational modification by O-GlcNAc: another way to change protein function. J. Cell Biochem. 98, 1062–1075 (2006).
Zeidan, Q. & Hart, G. W. The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J. Cell Sci. 123, 13–22 (2010).
Haltiwanger, R. S. & Philipsberg, G. A. Mitotic arrest with nocodazole induces selective changes in the level of O-linked N-acetylglucosamine and accumulation of incompletely processed N-glycans on proteins from HT29 cells. J. Biol. Chem. 272, 8752–8758 (1997).
Chou, C. F. & Omary, M. B. Mitotic arrest with anti-microtubule agents or okadaic acid is associated with increased glycoprotein terminal GlcNAc's. J. Cell Sci. 107, 1833–1843 (1994).
Hart, G. W., Housley, M. P. & Slawson, C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007).
Yang, W. H. et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 8, 1074–1083 (2006).
Love, D. C. & Hanover, J. A. The hexosamine signaling pathway: deciphering the 'O-GlcNAc code'. Sci. STKE 2005, re13 (2005).
Zachara, N. E. et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 (2004).
Ngoh, G. A. et al. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ. Res. 104, 41–49 (2009).
Slawson, C., Copeland, R. J. & Hart, G. W. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem. Sci. doi: 10.1016/j.tibs.2010.04.005 (in the press).
Wang, Z., Gucek, M. & Hart, G. W. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc. Natl Acad. Sci. USA 105, 13793–13798 (2008).
Gao, Y., Parker, G. J. & Hart, G. W. Streptozotocin-induced β-cell death is independent of its inhibition of O-GlcNAcase in pancreatic Min6 cells. Arch. Biochem. Biophys. 383, 296–302 (2000).
Vosseller, K., Wells, L., Lane, M. D. & Hart, G. W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl Acad. Sci. USA 99, 5313–5318 (2002).
Abe, M. & Oshima, R. G. A single human keratin 18 gene is expressed in diverse epithelial cells of transgenic mice. J. Cell Biol. 111, 1197–1206 (1990).
Ku, N. -O., Strnad, P., Zhong, B. H., Tao, G. Z. & Omary, M. B. Keratins let liver live: mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology 46, 1639–1649 (2007).
Gukovskaya, A. S., Gukovsky, I., Jung, Y., Mouria, M. & Pandol, S. J. Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J. Biol. Chem. 277, 22595–22604 (2002).
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Stambolic, V. & Woodgett, J. R. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol. 16, 461–466 (2006).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489–501 (2002).
Chen, W. S. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208 (2001).
Andjelkovic, M. et al. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272, 31515–31524 (1997).
Parker, P. J. & Murray-Rust, J. PKC at a glance. J. Cell Sci. 117, 131–132 (2004).
Liu, Y., Graham, C., Li, A., Fisher, R. J. & Shaw, S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem. J. 361, 255–265 (2002).
Hayashi, K. & Altman, A. Protein kinase C theta (PKCθ): a key player in T cell life and death. Pharmacol. Res. 55, 537–544 (2007).
Nadeau, S. I. & Landry, J. Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. Adv. Exp. Med. Biol. 594, 100–113 (2007).
Paramio, J. M., Segrelles, C., Ruiz, S. & Jorcano, J. L. Inhibition of protein kinase B (PKB) and PKCζ mediates keratin K10-induced cell cycle arrest. Mol. Cell Biol. 21, 7449–7459 (2001).
Ku, N. -O. et al. Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J. Cell Biol. 143, 2023–2032 (1998).
Ku, N. -O., Soetikno, R. M. & Omary, M. B. Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology 37, 1006–1014 (2003).
Ku, N. -O., Michie, S., Oshima, R. G. & Omary, M. B. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J. Cell Biol. 131, 1303–1314 (1995).
Magin, T. M. et al. Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J. Cell Biol. 140, 1441–1451 (1998).
Soesanto, Y. A. et al. Regulation of Akt signalling by O-GlcNAc in euglycemia. Am. J. Physiol. Endocrinol. Metab. 295, E974–980 (2008).
Dong, D. L., Xu, Z. S., Hart, G. W. & Cleveland, D. W. Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J. Biol. Chem. 271, 20845–20852 (1996).
Slawson, C., Lakshmanan, T., Knapp, S. & Hart, G. W. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol. Biol. Cell. 19, 4130–4140 (2008).
Ku, N. -O. et al. Studying simple epithelial keratins in cells and tissues. Methods Cell Biol. 78, 489–517 (2004).
Ku, N. -O., Liao, J. & Omary, M. B. Apoptosis generates stable fragments of human type I keratins. J. Biol. Chem. 272, 33197–33203 (1997).
Acknowledgements
We are grateful to R. Oshima (The Sanford-Burnham Medical Research Institute) and T. Magin (University of Leipzig) for making the mice that express wild-type K18 and the K18-null available to us, Y. Chen-Tsai and the Stanford University Transgenic Facility for helping generate the transgenic mice, E. Resurreccion for assistance with immune staining and P. Chu for assistance with hematoxylin and eosin staining. This work was supported by NIH grant DK47918 and the Department of Veterans Affairs (M.B.O.), NIH Digestive Disease Center grant DK56339 to Stanford University, NIH Michigan Gastrointestinal Peptide Research Center grant DK34933 and WCU project (R31-2008-000-10086-0) from the Korean Ministry of Education, Science and Technology to N. -O.K.
Author information
Authors and Affiliations
Contributions
N.-O.K. and M.B.O. conceived and designed the study. N.-O.K., D.M.T. and P.S. performed the experiments and analysed the data. N.-O.K., D.M.T., P.S. and M.B.O. interpreted the data. N.-O.K. and M.B.O. wrote the manuscript with comments from D.M.T. and P.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1609 kb)
Rights and permissions
About this article
Cite this article
Ku, NO., Toivola, D., Strnad, P. et al. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol 12, 876–885 (2010). https://doi.org/10.1038/ncb2091
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2091
This article is cited by
-
The apoptotic effects of soybean agglutinin were induced through three different signal pathways by down-regulating cytoskeleton proteins in IPEC-J2 cells
Scientific Reports (2023)
-
O-GlcNAcylation regulates neurofilament-light assembly and function and is perturbed by Charcot-Marie-Tooth disease mutations
Nature Communications (2023)
-
Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis
Molecular Cancer (2019)
-
Multifaceted role of keratins in epithelial cell differentiation and transformation
Journal of Biosciences (2019)
-
The role of keratins in the digestive system: lessons from transgenic mouse models
Histochemistry and Cell Biology (2018)