Abstract
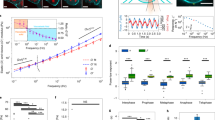
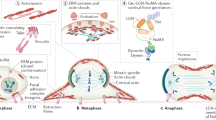
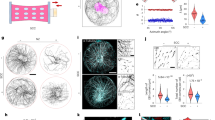
The response of cells to forces is essential for tissue morphogenesis and homeostasis. This response has been extensively investigated in interphase cells, but it remains unclear how forces affect dividing cells. We used a combination of micro-manipulation tools on human dividing cells to address the role of physical parameters of the micro-environment in controlling the cell division axis, a key element of tissue morphogenesis. We found that forces applied on the cell body direct spindle orientation during mitosis. We further show that external constraints induce a polarization of dynamic subcortical actin structures that correlate with spindle movements. We propose that cells divide according to cues provided by their mechanical micro-environment, aligning daughter cells with the external force field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gönczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 (2008).
Ahringer, J. Control of cell polarity and mitotic spindle positioning in animal cells. Curr. Opin. Cell Biol. 15, 73–81 (2003).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Lu, B., Roegiers, F., Jan, L. Y. & Jan, Y. N. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409, 522–525 (2001).
Fernandez-Minan, A., Martin-Bermudo, M. D. & Gonzalez-Reyes, A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 17, 683–688 (2007).
Toyoshima, F. & Nishida, E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 26, 1487–1498 (2007).
Thery, M. et al. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953 (2005).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006).
Terenna, C. R. et al. Physical mechanisms redirecting cell polarity and cell shape in fission yeast. Curr. Biol. 18, 1748–1753 (2008).
Farhadifar, R., Roper, J. C., Aigouy, B., Eaton, S. & Julicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Rauzi, M., Verant, P., Lecuit, T. & Lenne, P. F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401–1410 (2008).
Wozniak, M. A. & Chen, C. S. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 (2009).
Thery, M., Jimenez-Dalmaroni, A., Racine, V., Bornens, M. & Julicher, F. Experimental and theoretical study of mitotic spindle orientation. Nature 447, 493–496 (2007).
Cramer, L. P. & Mitchison, T. J. Myosin is involved in postmitotic cell spreading. J. Cell Biol. 131, 179–189 (1995).
Azioune, A., Storch, M., Bornens, M., Thery, M. & Piel, M. Simple and rapid process for single cell micro-patterning. Lab. on a chip 9, 1640–1642 (2009).
Burton, K. & Taylor, D. L. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature 385, 450–454 (1997).
Guck, J. et al. The optical stretcher: a novel laser tool to micromanipulate cells. Biophys. J. 81, 767–784 (2001).
Chartier, L. et al. Calyculin-A increases the level of protein phosphorylation and changes the shape of 3T3 fibroblasts. Cell Motil. Cytoskeleton 18, 26–40 (1991).
Stewart, M. P. et al. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature 469, 226–230 (2011).
Jungbauer, S., Gao, H., Spatz, J. P. & Kemkemer, R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 95, 3470–3478 (2008).
O’Connell, C. B. & Wang, Y. L. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell 11, 1765–1774 (2000).
Effler, J. C. et al. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr. Biol. 16, 1962–1967 (2006).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Burkel, B. M., von Dassow, G. & Bement, W. M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton 64, 822–832 (2007).
Mitsushima, M. et al. Revolving movement of a dynamic cluster of actin filaments during mitosis. J. Cell Biol. 191, 453–462 (2010).
Hamaguchi, M. S. & Hiramoto, Y. Analysis of the role of astral rays in pronuclear migration in sand dollar eggs by the colcemid-UV method. Dev. Growth Differ. 28, 143–156 (1986).
Minc, N., Burgess, D. & Chang, F. Influence of cell geometry on division-plane positioning. Cell 144, 414–426 (2011).
Wuhr, M., Tan, E. S., Parker, S. K., Detrich, H. W. 3rd & Mitchison, T. J. A model for cleavage plane determination in early amphibian and fish embryos. Curr. Biol. 20, 2040–2045 (2010).
Azoury, J. et al. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 (2008).
Schuh, M. & Ellenberg, J. A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 (2008).
Woolner, S., O’Brien, L. L., Wiese, C. & Bement, W. M. Myosin-10 and actin filaments are essential for mitotic spindle function. J. Cell Biol. 182, 77–88 (2008).
Yoshigi, M., Hoffman, L. M., Jensen, C. C., Yost, H. J. & Beckerle, M. C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 171, 209–215 (2005).
Woodard, G. E. et al. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol. Cell Biol. 30, 3519–3530 (2010).
Hamant, O. et al. Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655 (2008).
Ranft, J. et al. Fluidization of tissues by cell division and apoptosis. Proc. Natl Acad. Sci. USA 107, 20863–20868 (2010).
Azioune, A., Carpi, N., Tseng, Q., Thery, M. & Piel, M. Protein micropatterns: a direct printing protocol using deep UVs. Methods Cell Biol. 97, 133–146 (2010).
Tolic-Norrelykke, S. F. & Schaffer, E. Calibration of optical tweezers with positional detection in the back focal plane. Rev. Sci. Instrum. 77, 103101–103111 (2006).
Maniotis, A. J., Chen, C. S. & Ingber, D. E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. USA 94, 849–854 (1997).
Hertwig, O. Ueber den Werth der ersten Furchungszellen für die Organbildung des Embryo. Experimentelle Studien am Frosch-und Tritonei. Arch. Mikrosk. Anat. 42, 662–807 (1893).
Boulanger, J. et al. Patch-based nonlocal functional for denoising fluorescence microscopy image sequences. IEEE Trans. Med. Imaging 29, 442–454 (2010).
Acknowledgements
We are grateful to J. Colombelli for helpful discussions and preliminary experiments on retraction fibre cutting; L. Mahadevan for helpful discussions concerning mechanical cortex anisotropy; D. Gerlich (ETH, Zurich, Switzerland) and R. Tsien (UCSD, San Diego, USA) for the pIRES-puro3–MyrPalm–GFP plasmid; V. Doye (IJM, Paris, France) for the pIRES-neo–histone2B–mCherry plasmid; W. Bement (University of Wisconsin, Madison, USA) for the GFP–Utr-CH plasmid; R. Wedlich-Soldner (IMPRS, Martinsried, Germany) and G. Montagnac (Institut Curie, Paris, France) for the Lifeact–mCherry plasmid; M. Heuze (Institut Curie, Paris, France) and A. M. Lennon-Dumesnil (Institut Curie, Paris, France) for the Lifeact–mCherry lentivirus; V. Fraisier, the Nikon Imaging Center and the PICT–IBiSA of the Institut Curie for technical support in microscopy; J. Boulanger for image treatment using ndsafir; and Z. Maciarowski, C. Guérin and A. Viguier for FACS sorting of stable cell lines. We thank M. Thery and J. Aubertin for helpful discussions throughout the course of this work and A. W. Murray, E. Paluch, A. M. Lennon-Dumesnil, A. Taddei, M. Thery, S. Misery-Lenkei and members of the Piel laboratory for critical reading of the manuscript. This work was supported by the Centre National de la Recherche Scientifique, the Institut Curie and by ANR (ANR-06-PCVI-0010) and HFSP grants to M.P. J.F. was supported by pre-doctoral fellowships from Boehringer Ingelheim Fonds and the Association pour la Recherche sur le Cancer.
Author information
Authors and Affiliations
Contributions
J.F. designed, carried out and analysed most experiments and wrote the article, N.C. carried out most cell stretching experiments as well as experiments shown in Fig. 2f and Supplementary Fig. S3, T.B. carried out and analysed optical trap experiments (Fig. 2c–e), A.B. carried out some cell stretching experiments, M.C. carried out the experiments shown in Fig. 2f and Supplementary Fig. S3, A.A. developed the method to produce micropatterns on stretchable substrates, M.B. contributed ideas, discussion and supervised part of the work of J.F., C.S. contributed ideas and discussion and supervised the work on optical trap experiments, L.F. carried out laser ablation experiments (Fig. 1), D.C. set up the cell stretching device, supervised the work of A.B. and contributed ideas and discussion, and M.P. supervised the work, carried out experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1372 kb)
Supplementary Information
Supplementary Movie 1 (MOV 3276 kb)
Supplementary Information
Supplementary Movie 2 (MOV 5103 kb)
Supplementary Information
Supplementary Movie 3 (MOV 5099 kb)
Supplementary Information
Supplementary Movie 4 (MOV 6803 kb)
Supplementary Information
Supplementary Movie 5 (MOV 5944 kb)
Supplementary Information
Supplementary Movie 6 (MOV 4590 kb)
Supplementary Information
Supplementary Movie 7 (MOV 7568 kb)
Supplementary Information
Supplementary Movie 8 (MOV 7677 kb)
Rights and permissions
About this article
Cite this article
Fink, J., Carpi, N., Betz, T. et al. External forces control mitotic spindle positioning. Nat Cell Biol 13, 771–778 (2011). https://doi.org/10.1038/ncb2269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2269
This article is cited by
-
In mitosis integrins reduce adhesion to extracellular matrix and strengthen adhesion to adjacent cells
Nature Communications (2023)
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
-
CDK1–cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry
Nature Cell Biology (2022)
-
Spatiotemporal dynamics of single cell stiffness in the early developing ascidian chordate embryo
Communications Biology (2021)
-
Spindle positioning and its impact on vertebrate tissue architecture and cell fate
Nature Reviews Molecular Cell Biology (2021)