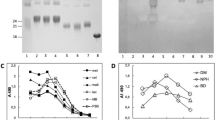
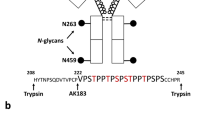
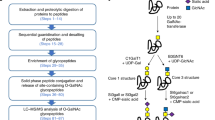
Abstract
Studies of post-translational modification by β-N-acetyl-D-glucosamine (O-GlcNAc) are hampered by a lack of efficient tools such as O-GlcNAc–specific antibodies that can be used for detection, isolation and site localization. We have obtained a large panel of O-GlcNAc–specific IgG monoclonal antibodies having a broad spectrum of binding partners by combining three-component immunogen methodology with hybridoma technology. Immunoprecipitation followed by large-scale shotgun proteomics led to the identification of more than 200 mammalian O-GlcNAc–modified proteins, including a large number of new glycoproteins. A substantial number of the glycoproteins were enriched by only one of the antibodies. This observation, combined with the results of inhibition ELISAs, suggests that the antibodies, in addition to their O-GlcNAc dependence, also appear to have different but overlapping local peptide determinants. The monoclonal antibodies made it possible to delineate differentially modified proteins of liver in response to trauma-hemorrhage and resuscitation in a rat model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hart, G.W., Housley, M.P. & Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007).
Wells, L., Vosseller, K. & Hart, G.W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291, 2376–2378 (2001).
Golks, A. & Guerini, D. The O-linked N-acetylglucosamine modification in cellular signalling and the immune system. 'Protein modifications: beyond the usual suspects' review series. EMBO Rep. 9, 748–753 (2008).
Lefebvre, T. et al. Does O-GlcNAc play a role in neurodegenerative diseases? Expert Rev. Proteomics 2, 265–275 (2005).
Dias, W.B. & Hart, G.W. O-GlcNAc modification in diabetes and Alzheimer's disease. Mol. Biosyst. 3, 766–772 (2007).
Copeland, R.J., Bullen, J.W. & Hart, G.W. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am. J. Physiol. Endocrinol. Metab. 295, E17–E28 (2008).
Laczy, B. et al. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 296, H13–H28 (2009).
Fulop, N., Zhang, Z., Marchase, R.B. & Chatham, J.C. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am. J. Physiol. Heart Circ. Physiol. 292, H2227–H2236 (2007).
Liu, J., Marchase, R.B. & Chatham, J.C. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am. J. Physiol. Heart Circ. Physiol. 293, H1391–H1399 (2007).
Champattanachai, V., Marchase, R.B. & Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am. J. Physiol. Cell Physiol. 294, C1509–C1520 (2008).
Rexach, J.E., Clark, P.M. & Hsieh-Wilson, L.C. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat. Chem. Biol. 4, 97–106 (2008).
Wang, Z. & Hart, G.W. Glycomic approaches to study GlcNAcylation: protein identification, site-mapping and site-specific O-GlcNAc quantification. Clin. Proteomics 4, 5–13 (2008).
Comer, F.I., Vosseller, K., Wells, L., Accavitti, M.A. & Hart, G.W. Characterization of a mouse monoclonal antibody specific for O-linked N-acetylglucosamine. Anal. Biochem. 293, 169–177 (2001).
Snow, C.M., Senior, A. & Gerace, L. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J. Cell Biol. 104, 1143–1156 (1987).
Kreppel, L.K. & Hart, G.W. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J. Biol. Chem. 274, 32015–32022 (1999).
Ingale, S., Wolfert, M.A., Gaekwad, J., Buskas, T. & Boons, G.J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 3, 663–667 (2007).
Haltiwanger, R.S., Grove, K. & Philipsberg, G.A. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J. Biol. Chem. 273, 3611–3617 (1998).
Wells, L., Whelan, S.A. & Hart, G.W. O-GlcNAc: a regulatory post-translational modification. Biochem. Biophys. Res. Commun. 302, 435–441 (2003).
Vosseller, K., Wells, L., Lane, M.D. & Hart, G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3–L1 adipocytes. Proc. Natl. Acad. Sci. USA 99, 5313–5318 (2002).
Dentin, R., Hedrick, S., Xie, J., Yates, J. III & Montminy, M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319, 1402–1405 (2008).
Yang, X. et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451, 964–969 (2008).
Zhang, F. et al. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 115, 715–725 (2003).
Ohn, T., Kedersha, N., Hickman, T., Tisdale, S. & Anderson, P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat. Cell Biol. 10, 1224–1231 (2008).
Chatham, J.C., Not, L.G., Fulop, N. & Marchase, R.B. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock 29, 431–440 (2008).
Viner, R.I., Zhang, T., Second, T. & Zabrouskov, V. Quantification of post-translationally modified peptides of bovine alpha-crystallin using tandem mass tags and electron transfer dissociation. J. Proteomics 72, 874–885 (2009).
Khidekel, N. et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat. Chem. Biol. 3, 339–348 (2007).
Clark, P.M. et al. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J. Am. Chem. Soc. 130, 11576–11577 (2008).
Chalkley, R.J., Thalhammer, A., Schoepfer, R. & Burlingame, A.L. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc. Natl. Acad. Sci. USA 106, 8894–8899 (2009).
Zachara, N.E. et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 (2004).
Guinez, C. et al. Hsp70-GlcNAc-binding activity is released by stress, proteasome inhibition, and protein misfolding. Biochem. Biophys. Res. Commun. 361, 414–420 (2007).
Ramirez-Correa, G.A. et al. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ. Res. 103, 1354–1358 (2008).
Hu, Y. et al. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J. Biol. Chem. 284, 547–555 (2009).
Wang, Y., Vera, L., Fischer, W.H. & Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460, 534–537 (2009).
Bialik, S. & Kimchi, A. The death-associated protein kinases: structure, function, and beyond. Annu. Rev. Biochem. 75, 189–210 (2006).
Not, L.G., Marchase, R.B., Fulop, N., Brocks, C.A. & Chatham, J.C. Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats. Shock 28, 345–352 (2007).
Lim, J.M. et al. Defining the regulated secreted proteome of rodent adipocytes upon the induction of insulin resistance. J. Proteome Res. 7, 1251–1263 (2008).
Acknowledgements
We thank R. Davis (University of Georgia) for monoclonal antibody production, J.-M. Lim and L. Zhao for expert assistance with mass spectrometry, E.G. El-Karim for assistance with molecular biology and T. Buskas for assistance with glycopeptide synthesis and protein conjugation. We also thank BioInquire, Inc. for access to the beta version of ProteoIQ that was used in the evaluation of the mass spectrometry data. We thank G.W. Hart (Johns Hopkins School of Medicine) for CTD110.6 and AL28 (anti-OGT antibodies) and S.W. Whiteheart (University of Kentucky) for the anti-OGA antibody. This research was supported by a grant from the US National Institute of Diabetes and Digestive and Kidney disorders (NIH RO1 DK075069 to L.W.), the Research Resource for Integrated Glycotechnology (NIH/NCRR P41R005351 to G.-J.B.) and the National Cancer Institute of the US National Institutes of Health (NIH/NCI R01CA088986 to G.-J.B.). C.F.T. was supported by a predoctoral fellowship from the American Heart Association (Southeast Affiliation). This work was further supported by grants NIH HL067464 and HL079364 (to J.C.C.).
Author information
Authors and Affiliations
Contributions
S.I. and G.A.E. performed the chemical synthesis. M.A.W. performed, analyzed and directed the immunological experiments. C.F.T. performed and analyzed the western blots and MS experiments. L.G.N. and J.C.C. were responsible for the rat model. L.W. and G.-J.B. were responsible for the overall experimental design and wrote the paper. G.-J.B. was responsible for compound design.
Corresponding authors
Ethics declarations
Competing interests
The University of Georgia Research Foundation, Inc. has non-exclusively licensed the antibodies to Millipore Corporation.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–6 and Supplementary Tables 1–7 (PDF 3767 kb)
Rights and permissions
About this article
Cite this article
Teo, C., Ingale, S., Wolfert, M. et al. Glycopeptide-specific monoclonal antibodies suggest new roles for O-GlcNAc. Nat Chem Biol 6, 338–343 (2010). https://doi.org/10.1038/nchembio.338
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.338
This article is cited by
-
An intellectual disability syndrome with single-nucleotide variants in O-GlcNAc transferase
European Journal of Human Genetics (2020)
-
Investigation of post-translational modifications in type 2 diabetes
Clinical Proteomics (2018)
-
Genome-wide chemical mapping of O-GlcNAcylated proteins in Drosophila melanogaster
Nature Chemical Biology (2017)
-
A mutant O-GlcNAcase enriches Drosophila developmental regulators
Nature Chemical Biology (2017)
-
O-GlcNAc profiling: from proteins to proteomes
Clinical Proteomics (2014)