Abstract
Regulatory T (Treg) cells are important for the maintenance of immune homoeostasis and prevention of autoimmune diseases. Epigenetic modifications have been reported to modulate autoimmunity by altering Treg cell fate. Here we show that the H3K4 methyltransferase Ash1l facilitates TGF-β-induced Treg cell polarization in vitro and protects mice from T cell-mediated colitis in vivo. Ash1l upregulates Smad3 expression by directly targeting Smad3 promoter to increase local H3K4 trimethylation. Furthermore, we identify an lncRNA, namely lnc-Smad3, which interacts with the histone deacetylase HDAC1 and silences Smad3 transcription. After TGF-β stimulation, activated Smad3 suppresses lnc-Smad3 transcription, thereby recovering the Smad3 promoter accessibility to Ash1l. By revealing the opposite regulatory functions of Ash1l and lnc-Smad3 in Smad3 expression, our data provide insights for the epigenetic control of Treg cell fate to potentially aid in the development of therapeutic intervention for autoimmune diseases.
Similar content being viewed by others
Introduction
Regulatory T (Treg) cells are essential for immune homoeostasis by suppressing effector T cell responses during infection, inflammation and autoimmunity1,2. Cytokines, particularly transforming growth factor-β (TGF-β) have essential functions for the induction of Foxp3 expression and polarization of Treg cells3,4,5. TGF-β signalling triggers the phosphorylation, activation and nuclear translocation of Smad proteins, such as Smad2 and Smad3, and then the activated Smad complex binds to the Foxp3 locus and promotes its expression, subsequently leading to Treg cell polarization6,7. How the TGF-β pathway is regulated to mediate Treg cell development needs further investigation. The mechanisms by which Smad proteins are epigenetically regulated are also poorly understood.
In addition to the well-established functions of transcription factors and cytokines in Treg cell development, other cues, such as epigenetic modifications, are also involved in Treg cell fate8,9. Previous studies show that Treg cells have a histone H3 lysine 27 (H3K27) trimethylation (H3K27me3) landscape distinct from that of naive T cells and other T helper cells, and appropriate histone modifications induced by activated Smad3 result in the promotion and stabilization of Foxp3 expression10,11. These studies highlight the involvement of epigenetic modifications in Treg cell development. Characterization of the detailed functions of epigenetic factors in Treg cell-mediated immune tolerance may thus be important for the development of potential interventions for inflammatory autoimmune disease.
Lysine methylation is one of the most characterized histone modifications to date. In particular, H3K4 methylation associated with transcriptional activation is critical for the maintenance of cell fates12. In our previous study, the screening of 14 known H3K4 methyltransferases and demethylases for their functions in regulating innate inflammatory immune find ([absent, small, or homeotic]-like [Drosophila]) Ash1l, a H3K4 methyltransferase, as a potent inhibitor of LPS-induced IL-6 production in macrophages13. We demonstrate that Ash1l inhibits TLR-triggered innate inflammatory response in macrophages by directly targeting Tnfaip3 promoter region to induce its expression. Ash1l-silenced mice are more susceptible to bacterial infection and autoimmune diseases13.
Ash1l is highly expressed in CD4+ T cells13. Exogenous expression of the plant homeodomain-like zinc finger domain of Ash1l inhibits the development of CD4+CD8+ thymocytes in vitro, suggesting that Ash1l may regulate early thymic T cell development14. In the gut, gene profiling data show that Ash1l is downregulated in colon tissues during T cell-mediated colitis in mice (GSE27302)15, and has lower (−2.9-fold) expression in epithelial biopsy samples of inflamed ascending colon than that in uninflamed sigmoid colon of ulcerative colitis patients (GSE11223)16. Lastly, Ash1l gene also constitutes part of the Idd17 locus associated with increased susceptibility to autoimmune diabetes in NOD mice17. Combined, these data indicate a potential connection between Ash1l and T cell-mediated autoimmune disease.
In this study, we show that Ash1l upregulates Smad3 expression by directly activating its promoter, and thereby promoting Foxp3 expression and induced Treg (iTreg) cell differentiation in vitro. By contrast, a newly-identified long non-coding RNA (lncRNA), lnc-Smad3, recruits HDAC1 to the Smad3 promoter and selectively suppresses Smad3 but not Foxp3 expression. Interestingly, activation of the TGF-β/Smad3 axis suppresses lnc-Smad3 transcription, restoring accessibility of the Smad3 promoter to Ash1l. Ash1l-silenced mice are more susceptible to T cell-mediated colitis due to the impairment of Treg cell polarization. Lastly, ASH1L, FOXP3 and SMAD3 are downregulated in peripheral CD4+ T cells from patients with rheumatoid arthritis. Our results provide insights for the epigenetic control of Treg cell polarization during immune homoeostasis, and suggest a possible association between Ash1l and immune disorders.
Results
Ash1l enhances Treg cell polarization by upregulating Foxp3
In our previous study, we already observed preferential expression of Ash1l in CD4+ T cells, which inspired us to further analyse the function of Ash1l in T cell development and differentiation13. We first analysed the phenotype and proportion of major T cell subsets in Ash1l-silenced mice, which were generated via inserting the PB transposon between exons 15 and 16 of Ash1l allele as compared with wild-type (WT) mice13. As shown in our previous study, the frequency of CD4+ and CD8+ T cells in splenocytes from Ash1l-silenced mice was normal13. The proportions of CD4+ single-positive (CD4SP) and CD8SP thymocytes, activated CD44hiCD62Llo CD4+ T cells in the spleen and mesenteric lymph nodes (mLN) were also normal in Ash1l-silenced mice (Supplementary Fig. 1a–d). Ash1l-silenced mice also had similar frequencies of Foxp3+ Treg in CD4SP thymocytes and peripheral CD4+ T cells from spleen and mLN (Supplementary Fig. 1e,f). Thus, silencing of Ash1l does not affect T cell development.
We then cultured naive CD4+ T cells from WT or Ash1l-silenced splenocytes under Th1, Th17 or induced Treg (iTreg) cell-skewing conditions to analyse the function of Ash1l in differentiation of CD4+ T cell subsets. Notably, we observed significantly impaired iTreg cell differentiation, but increased Th1 and Th17 differentiation in Ash1l-silenced CD4+ T cells as compared with WT CD4+ T cells (Supplementary Fig. 2a,b). In addition, naive CD4+ T cells from Ash1l-silenced mLN or CD4SP thymocytes also showed considerably attenuated induction of iTreg cells (Supplementary Fig. 2c,d). We also found that the expression of Ash1l did not change during iTreg cell differentiation and was also similar among different CD4+ T cell subsets, including naive CD4+ T cells, Th1, Th17 and iTreg cells (Supplementary Fig. 3a,b), indicating that Ash1l expression in CD4+ T cells may not be regulated during iTreg or T helper cell differentiation. In addition, we found that naive CD4+ T cells from WT or Ash1l-silenced splenocytes showed similar expression levels of the other H3K4 methyltransferases (Supplementary Fig. 3c), indicating that the deficiency of Ash1l does not affect the expression of other H3K4 methyltransferases.
Since Treg cells are defined by expression of transcriptional factor Foxp3, which is critical for the maintenance and function of Treg cells, we next examined the effect of Ash11 on Foxp3 expression and the function of iTreg. The expression of Foxp3 (Fig. 1a–c) was significantly reduced in Ash1l-silenced splenic CD4+ T cells under iTreg cell-skewing conditions (with TGF-β). Accordingly, Ash1l-silenced Foxp3+ iTreg cells showed lower suppressive functions, with reduced expression of costimulatory molecules CD25, CTLA-4 and GITR on iTreg cells (Supplementary Fig. 4a) and attenuated capacity of suppressing the proliferation of WT effector T cells (Supplementary Fig. 4b,c). Altogether, these data suggest that Ash1l is critical for TGF-β-mediated induction and activation of iTreg cells by promoting Foxp3 expression in vitro.
(a,b) Representative flow cytometry (a) and quantification (b) of the percentage of Foxp3 in the CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for indicated times. (c) mRNA expression of Foxp3 in the WT and Ash1l-silenced CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for indicated times. (d,e) Representative flow cytometry (d) and quantification (e) of the percentage of Foxp3 in WT and Ash1l-silenced CD4+ T cells transduced with Ash1l-fragment-expressing lentivirus (Lenti-Ash1l-F1, Lenti-Ash1l-F2, Lenti-Ash1l-F3 or Lenti-Ash1l-ΔN) and cultured under iTreg cell-skewing conditions (with TGF-β) for 3 days. (f) mRNA expression of Foxp3 in WT and Ash1l-silenced CD4+ T cells transduced and cultured as in d. Error bars represent s.d. Student’s t test. *P<0.05, **P<0.01. Data are representative of three independent experiments (a,d) or are from three independent experiments (b,c,e,f; mean±s.d.).
We next utilized lentivirus-mediated overexpression of Ash1l to confirm the positive effect of Ash1l on the induction of Foxp3 expression and iTreg cells. Considering the nearly 9 kb cDNA length of mouse Ash1l, we constructed Ash1l-fragment 1, 2, 3 (Ash1l-F1, -F2 and -F3) lentiviruses expressing 1–880 amino acids (aa), 881–1,855 aa and 1,886–2,958 aa containing SET (Su[var]3-9, E[z] and trithorax) domain of Ash1l, respectively. We previously showed that the single amino acid point mutation Ash1llΔN (N2212I) had abolished H3K4 methyltransferase activity13, so we also constructed Ash1lΔN (N2212I)-expressing lentivirus (Ash1l-ΔN). We observed that only overexpression of lentivirus Ash1l-F3 containing SET domain and normal H3K4 methyltransferase activity could enhance Foxp3 expression and iTreg cell polarization, rescuing the induction of Foxp3 and iTreg cells, which were impaired in Ash1l-silenced CD4+ T cells (Fig. 1d–f). Collectively, these data indicate that Ash1l promotes TGF-β-induced Foxp3 expression and iTreg cell polarization dependent on its SET domain-mediated H3K4 methyltransferase activity.
Ash1l-silenced mice are more susceptible to colitis
Treg cells are essential for prevention of inflammatory diseases and maintenance of immune homoeostasis. Next, we sought to investigate whether Ash1l was involved in regulation of T cell-mediated autoimmunity in vivo. We first investigated the susceptibility of Ash1l-silenced mice to the induction of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. Compared to WT mice, Ash1l-silenced mice developed more severe colitis upon TNBS induction, with a sharper loss of body weight, more exaggerated shortening of colon in appearance, and more severe colon inflammation as indicated by extensive infiltration of mononuclear cells, reduction of goblet cells and mucosa erosion in histology (Fig. 2a–c; Supplementary Fig. 5a). Moreover, upon TNBS induction, Ash1l-silenced mice had fewer Foxp3+ Treg cells in the spleen and mLN (Supplementary Fig. 5b), indicating that Ash1l could promote Treg cell differentiation in vivo under pathological conditions, which is critical for the prevention of overall inflammatory responses in colitis.
(a–c) TNBS-colitis was induced in 8-week-old WT and Ash1l-silenced mice (n=5 mice per group) and monitored for disease development for 3 days after TNBS induction. (a) Weight change over time of WT and Ash1l-silenced mice after TNBS induction, presented relative to weight at day 0, set as 100%. (b,c) Appearances (b) of colons, reduction of colon length (c) were examined at day3 after TNBS induction. (d–f) T cell adoptive transfer-mediated colitis was induced in 6-week-old Rag1−/− mice via transferring with WT CD4+CD25− T cells with or without WT or Ash1l-silenced iTreg cells (n=5 mice per group). Disease development was monitored for 6 weeks. (d) Weight change over time of Rag1−/− mice transferred with WT CD4+CD25− T cells with or without WT or Ash1l-silenced iTreg cells, presented relative to weight at week 1, set as 100%. (e,f) Appearances (e) and length (f) of colons were examined at week 6 after T cell transfer. Error bars represent s.d. Student’s t test. *P<0.05, **P<0.01. Data are from three independent experiments (a,c,d,f; mean±s.d. of five mice) or are representative of three independent experiments (b,e).
To further confirm whether Ash1l contributes to the prevention of colitis via modulating Treg cell function in vivo, we used a T cell adoptive transfer model of chronic colitis18. WT CD4+CD45RBhi T cells were transferred into Rag1−/− mice together with either WT or Ash1l-silenced in vitro differentiated iTreg cells. While WT iTreg cells efficiently protected mice from colitis, Ash1l-silenced iTreg cells failed to inhibit intestinal inflammation and weight loss (Fig. 2d–f; Supplementary Fig. 5c). We also tracked the fate of naive CD4+ T cells from WT and Ash1l-silenced mice upon adoptive transfer into Rag1−/− hosts. Compared to WT CD4+ T cells, fewer Ash1l-silenced CD4+ T cells converted into Foxp3+ iTreg cells (Supplementary Fig. 5d,e), which demonstrated that Ash1l promoted iTreg cell polarization in vivo.
Altogether, these data indicate that Ash1l prevents the development of T cell-mediated colitis by upregulating Treg cell polarization and function in vivo.
Ash1l enhances Smad2/3-TGF-β signalling via its SET domain
The induction of iTreg cells is mainly mediated by the TGF-β-Smad signalling. To further investigate the mechanism of Ash1l-mediated promotion of iTreg cell polarization, we examined the expression and activation of Smad proteins in WT and Ash1l-silenced CD4+ T cells upon TGF-β stimulation. Compared to WT CD4+ T cells, lower mRNA levels of Smad2 and Smad3 were observed in Ash1l-silenced CD4+ T cells under iTreg cell-skewing condition (Fig. 3a). With ELISA assay, we also observed less Smad2/Smad3 expression in whole-cell extract and nuclear extract of Ash1l-silenced CD4+ T cells during iTreg cell polarization, further indicating impaired induction and activation of Smad2 and Smad3 (Fig. 3b). Thus, Ash1l promotes TGF-β-induced Foxp3 expression through enhancing TGF-β-Smad2/3 signalling.
(a) mRNA expression of Smad2 (left) and Smad3 (right) in the WT and Ash1l-silenced CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for indicated times. Results are relative to those in unstimulated WT CD4+ T cells, set as 1. (b) ELISA assay of Smad2/3 in 5 μg whole-cell extract (left) and 5 μg nuclear extract (right) of WT and Ash1l-silenced CD4+ T cells under iTreg cell-skewing conditions (with TGF-β) for indicated times. OD450 represents absorbance at 450 nm. (c) mRNA expression of Smad2 and Smad3 in the WT and Ash1l-silenced CD4+ T cells transduced with a control lentivirus (Lenti-CTR) or Ash1l-fragment-expressing lentivirus (Lenti-Ash1l-F1, Lenti-Ash1l-F2, Lenti-Ash1l-F3 or Lenti-Ash1l-ΔN) and cultured under iTreg cell-skewing conditions (with TGF-β) for 3 days. Results are relative to those in WT CD4+ T cells transduced with Lenti-CTR, set as 1. (d) ELISA assay of Smad2/3 in 5 μg whole-cell extract (left) and 5 μg nuclear extract (right) of WT and Ash1l-silenced CD4+ T cells transduced and cultured as in c. OD450 represents absorbance at 450 nm. Error bars represent s.d. Student’s t test. *P<0.05, **P<0.01. All data are from three independent experiments (mean±s.d. of technical triplicates).
Then, we observed that only overexpression of lentivirus Ash1l-F3 could increase the expression of Smad2 and Smad3, rescuing the impairment of Smad2/3 activation in Ash1l-silenced CD4+ T cells (Fig. 3c,d). The data indicated that Ash1l indeed facilitates TGF-β-Smad2/3 signalling via enhancing Smad2/3 expression dependent on its H3K4 methyltransferase activity of SET domain.
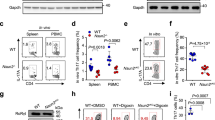
The above data promoted us to investigate the expression of ASH1L, FOXP3 and SMAD3 in T cells of the patients with inflammatory diseases. Indeed, ASH1L, FOXP3 and SMAD3 were significantly downregulated in CD4+ T cells from peripheral blood mononuclear cells (PBMCs) of patients with rheumatoid arthritis as compared to healthy controls (Supplementary Fig. 6; Supplementary Table 1), which indicates that ASH1L and ASH1L-mediated SMAD3/FOXP3 regulation might have potential significance in human autoimmune pathogenesis.
Ash1l targets Smad2/3 promoters to induce Smad2/3 expression
Since Ash1l is a histone methyltransferase that functions to promote gene transcription13, we investigated whether Ash1l could directly upregulate Smad2/3 expression at transcriptional level. Chromatin immunoprecipitation (CHIP) experiments with antibody to Ash1l revealed that Ash1l was enriched at the Smad2 and Smad3 promoter regions in iTreg cells (Fig. 4a). Next, we assessed the H3K4 trimethylation (H3K4me3) modification at Smad2 and Smad3 promoter regions in Ash1l-silenced and WT CD4+ T cells under iTreg cell-skewing condition. CHIP experiments with antibody to H3K4me3 revealed that TGF-β-induced upregulation of H3K4me3 modification at the promoter regions of Smad2 and Smad3 was attenuated in Ash1l-silenced CD4+ T cells (Fig. 4b). And we observed that the enrichment of Ash1l at Smad3 promoters was more potently upregulated by TGF-β stimulation than that at Smad2 promoters (Fig. 4a). Similar to Ash1l-silenced mice, Smad3-KO mice showed normal natural T cell development but impaired iTreg cell polarization (Supplementary Fig. 7a–f).
(a) CHIP analysis of the recruitment of Ash1l to the Smad2 and Smad3 promoter regions with anti-Ash1l antibody in CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for indicated times. Normalized data are shown as percentage of input control (% Inp). IgG serves as a CHIP control. (b) H3K4me3 modifications of the Smad2 and Smad3 promoter regions. CHIP analysis of the trimethylation of histone H3 lysine 4 (H3K4me3) at the Smad2 and Smad3 promoter regions in WT and Ash1l-silenced CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for 1 day. Normalized data are shown as percentage of input control (% Inp). IgG serves as a CHIP control. Error bars represent s.d. Student’s t test. *P<0.05, **P<0.01. All data are from three independent experiments (mean±s.d. of technical triplicates).
In contrast, the level of Smad4 was comparable between Ash1l-silenced and WT CD4+ T cells during iTreg cell polarization (Supplementary Fig. 8a). Consistently, we did not observe enrichment of Ash1l at the Smad4 and Foxp3 promoter regions in iTreg cells, nor did we see altered H3K4me3 modification at the Smad4 and Foxp3 promoter regions in Ash1l-silenced CD4+ T cells under iTreg cell-skewing condition (Supplementary Fig. 8b–e).
These data indicate that upon TGF-β stimulation, Ash1l selectively targets Smad2 and Smad3 promoter regions, and mediates H3K4me3 modification to enhance the expression of Smad2 and Smad3, thus strengthens TGF-β signalling.
Smad3 suppresses lnc-smad3 to promote iTreg polarization
The data presented above confirmed that Ash1l directly enhanced Smad2/3 expression, next we tried to give some cues for explaining the Ash1l-mediated selective regulation on Smad proteins. Considering the more TGF-β-induced enrichment of Ash1l at Smad3 promoter than that at Smad2 promoter, so we focused on explaining the selective promotion on Smad3 expression by Ash1l.
lncRNAs have been increasingly shown to regulate diverse biological processes including T cell development through affecting the expression of neighbouring genes19. A recent study catalogued the lncRNA expression profiles in various T cell types including iTreg cells20. So, we sought to determine whether there were any unrecognized lncRNAs proximal to the Smad3 gene locus that might be involved in Ash1l-mediated selective regulation of Smad3 expression and iTreg cell polarization. By analysing the gene loci nearby Smad3 locus from UCSC genome browser, we found an lncRNA (Gene symbol GM16759) located 165 kb upstream of Smad3 (Fig. 5a). We named this new lncRNA lnc-Smad3 because of its location and function verified as follows. We identified its sequence with 1,260 nucleotides (nt). Like most identified lncRNAs, lnc-Smad3 had 5′ cap structure and polyA tail, but had no protein coding capacity (Supplementary Fig. 9a,b). Lnc-Smad3 was expressed highly in CD4+ T cells and B cells, but extremely lowly in other immune cells such as CD8+ T cells and iTreg cells (Supplementary Fig. 9c). In addition, lnc-Smad3 was mainly located in the nucleus in CD4+ T cells, with a small portion existed in the cytoplasm (Supplementary Fig. 9d,e).
(a) A schematic outlining the genomic loci of lnc-Smad3 and Smad3. Semi-quantitative PCR (b) and gel electrophoresis (c) detection of lnc-Smad3 in CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for indicated times. Results are relative to the baseline lnc-Smad3 expression in unstimulated CD4+ T cells, set as 1. (d) Relative expression of lnc-Smad3 in WT and Smad3-KO CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for 3 days. Results are relative to the lnc-Smad3 expression in unstimulated WT CD4+ T cells, set as 1. (e) CHIP analysis of the accumulation of Smad3 at the lnc-Smad3 promoter regions with anti-Smad3 antibody in CD4+ T cells stimulated under iTreg cell-skewing conditions (with TGF-β) for 2 days. Normalized data are shown as percentage of input control (% Inp). IgG serves as a CHIP control. (f) Relative expression of lnc-Smad3, Smad3 and Foxp3 in CD4+ T cells transduced with a control lentivirus (Lenti-CTR) or lnc-Smad3-expressing lentivirus (Lenti-lnc-Smad3) and cultured under iTreg cell-skewing conditions (with TGF-β) for 3 days. Results are relative to those in CD4+ T cells transduced with Lenti-CTR, set as 1. (g,h) The percentages of Foxp3+ Tregs in CD4+ T cells transduced and cultured as in f were analysed by flow cytometry (g) and quantified (h). Numbers in quadrants indicate per cent cells in each. Error bars represent s.d. Student’s t test. NS, not significant. *P<0.05, **P<0.01. Data are from three independent experiments (b,d-f,h; mean±s.d. of technical triplicates) or are representative of three independent experiments (c,g).
In contrast to the upregulation of Smad3 by TGF-β (Fig. 3a,b), the expression of lnc-Smad3 was gradually reduced after TGF-β stimulation in CD4+ T cells and B cells (Fig. 5b,c; Supplementary Fig. 9f). The inverse correlation between lnc-Smad3 and Smad3 expression during iTreg cell polarization and close location of these two genes prompted us to determine whether lnc-Smad3 and Smad3 regulate each other’s expression. Since previous studies demonstrated Smad3 suppressed expression of some TGF-β-responsive genes via binding to their promoter regions21, we suspected Smad3 might directly target lnc-Smad3 promoter region to inhibit its expression as a transcriptional suppressor. Indeed, Smad3-deficient CD4+ T cells had significantly increased lnc-Smad3 expression than WT CD4+ T cells, both before and more significantly after TGF-β administration, indicating that Smad3 negatively regulates lnc-Smad3 expression (Fig. 5d). CHIP assays with antibody to Smad3 revealed that TGF-β induced Smad3 accumulation at the lnc-Smad3 promoter region (Fig. 5e). These results indicated that Smad3 acts as a transcriptional suppressor of lnc-Smad3 in TGF-β-mediated iTreg cells by directly targeting the lnc-Smad3 promoter region.
Next, we investigated the potential function of lnc-Smad3 in TGF-β-induced Smad3 and Foxp3 expression and iTreg cell differentiation. Upon TGF-β stimulation, overexpression of lnc-Smad3 led to a reduction of Smad3 and Foxp3 expression and impairment in the iTreg cell polarization in CD4+ T cells (Fig. 5f–h). However, knockdown of lnc-Smad3 in activated CD4+ T cells neither affected the expression of Smad3 and Foxp3 nor polarized iTreg cell polarization without TGF-β stimulation (Supplementary Fig. 10a–c), thus the reduction of lnc-Smad3 is required for iTreg cell polarization in a manner dependent on TGF-β. Altogether, TGF-β-induced Smad3 inhibits lnc-Smad3 expression via binding to its promoter region as a suppressor, and downregulation of lnc-Smad3 mediated by TGF-β consequently skews CD4+ T cells towards iTreg cells via increasing Smad3 and Foxp3 expression.
Lnc-Smad3 prevents Ash1l binding to the Smad3 promoter
Given the inverse expression of lnc-Smad3 and Smad3 during iTreg cell polarization, we next examined the epigenetic profile in lnc-Smad3 and Smad3 loci. With CHIP experiments, we found that with TGF-β stimulation, the H3K4me3 and H3K27 acetylation (H3K27ac), and the occupancy of Pol II were downregulated at lnc-Smad3 promoter region whereas these parameters were upregulated at the Smad3 promoter region (Fig. 6a). This was consistent with the downregulation of lnc-Smad3 and upregulation of Smad3 expression during iTreg cell polarization.
(a) CHIP analysis of the H3K4me3 and H3K27ac modifications, and Pol II occupancy around lnc-Smad3 and Smad3 loci in CD4+ T cells left unstimulated (day 0) or cultured under iTreg cell-skewing conditions (with TGF-β) for 2 days (day 2). Four regions (capital letters A–D) across lnc-Smad3 gene locus and three regions (capital letters E–G) across Smad3 gene locus were analysed by CHIP assay. Normalized data are shown as percentage of input control. (b) HepG2 cells were transfected with a Smad3 promoter reporter construct (1 kb upstream of the transcription start site) and lnc-Smad3 expression vector (lnc-Smad3) or empty control vector (CTR), and stimulated with TGF-β. Luciferase activity was measured 48 h later. Data were normalized to renilla luciferase and presented with respect to CTR, set as 1. (c) Chromatin accessibility of the Smad3 promoter region by quantitative PCR with DNase I pretreated nucleus of CD4+ T cells transduced with a control lentivirus (Lenti-CTR) or lnc-Smad3-expressing lentivirus (Lenti-lnc-Smad3) and cultured under iTreg cell-skewing conditions (with TGF-β) for 2 days. Changed fold are concluded using 2ΔCt with respect to CD4+ T cells transduced with Lenti-CTR, set as 1. (d) CHIP analysis of the accumulation of Ash1l and H3K4me3 modification at Smad3 promoter regions in CD4+ T cells transduced and cultured as in c. Normalized data are shown as percentage of input control (% Inp). IgG serves as a CHIP control. Error bars represent s.d. Student’s t test. *P<0.05, **P<0.01. All data are from three independent experiments (mean±s.d. of technical triplicates).
Above data suggested that Ash1l and lnc-Smad3 mediated opposing regulation of Smad3 expression, so we sought to determine the underlying mechanisms how Ash1l and lnc-Smad3 established an epigenetic balance between inhibition and activation of Smad3 transcription. Since lncRNAs usually regulate neighbouring genes by recruiting specific chromatin remodelers19, we performed RNA-immunoprecipitation (RIP) assay to assess the interaction between lnc-Smad3 and Smad3 or Ash1l. However, we observed no obvious interactions either between lnc-Smad3 and Smad3 or between lnc-Smad3 and Ash1l, indicating that lnc-Smad3 regulates Smad3 expression independently on the direct interaction with Smad3 or Ash1l (Supplementary Fig. 11a). To determine the direct effect of lnc-Smad3 in regulating Smad3 promoter, we transfected HepG2 cells with lnc-Smad3 expression vector or empty control vector, as well as Smad3 promoter luciferase-reporter construct under TGF-β stimulation. The luciferase activity was lower in the presence of exogenous lnc-Smad3 than that with empty control (Fig. 6b), indicating that lnc-Smad3 suppresses the activity of Smad3 promoter.
Notably, a DNase I sensitivity assay revealed that the chromatin accessibility of the Smad3 promoter was significantly attenuated in TGF-β-stimulated CD4+ T cells upon overexpression of lnc-Smad3 (Fig. 6c), indicating that lnc-Smad3 was involved in maintenance of the compact chromatin structure of the Smad3 promoter. We went further to determine whether lnc-Smad3 regulates the binding of the Smad3 promoter by Ash1l. With CHIP experiments using antibodies to Ash1l and H3K4me3, we found that overexpression of lnc-Smad3 in CD4+ T cells under TGF-β stimulation reduced the binding of Ash1l at the Smad3 promoter region, and hence attenuated its H3K4me3 modification (Fig. 6d). In contrast, the chromatin accessibility, Ash1l binding and H3K4me3 modification of the Foxp3 promoter were not altered by overexpression of lnc-Smad3 (Supplementary Fig. 11b,c), indicating that lnc-Smad3 selectively regulated the chromatin state of Smad3 but not Foxp3 promoter region.
Next, we tried to determine how lnc-Smad3 regulated the chromatin state of Smad3 promoter region. Previous research demonstrated that HDAC1, a histone deacetylase, could bind to Smad3 promoter region in lung cancer cells to suppress Smad3 expression in human lung cancer cells22. So, we investigated whether HDAC1 may have similar effect in mouse CD4+ T cells to close the chromatin structure of Smad3 promoter. By homologous comparison, we found that the promoter regions of Smad3 were highly conserved between human and mouse (Fig. 7a). With CHIP experiments using antibody to HDAC1, we found HDAC1 bound to Smad3 promoter in mouse naive CD4+ T cells, and TGF-β stimulation, which led to reduction of lnc-Smad3 expression (Fig. 5b), also prevented the accumulation of HDAC1 to Smad3 promoter (Fig. 7b). With RIP assay, we observed the interaction between HDAC1 and lnc-Smad3 in naive CD4+ T cells (Fig. 7c). Furthermore, overexpression of lnc-Smad3 in CD4+ T cells under TGF-β stimulation increased the binding of HDACl at the Smad3 promoter region (Fig. 7d) without affecting the HDAC1 expression (Fig. 7e). Thus, lnc-Smad3 selectively promoted the closed chromatin state of the Smad3 promoter by recruiting HDAC1.
(a) A schematic outlining the conserved sequences of the Smad3 promoter regions in Mus musculus and Homo sapiens. (b) CHIP analysis of the accumulation of HDAC1 at Smad3 promoter region in naive CD4+ T cells and iTreg cells. Normalized data are shown as percentage of input control (% Inp). IgG serves as a CHIP control. (c) Quantitative PCR detection of the lnc-Smad3 retrieved by HDAC1 specific antibody compared with immunoglobulin G (IgG) in the RIP assay within CD4+ T cells. Normalized data are shown as percentage of input control (% Inp). IgG serves as a RIP control. (d) CHIP analysis of the accumulation of HDAC1 at Smad3 promoter region in CD4+ T cells transduced with a control lentivirus (Lenti-CTR) or lnc-Smad3-expressing lentivirus (Lenti-lnc-Smad3) and cultured under iTreg cell-skewing conditions (with TGF-β) for 2 days. Normalized data are shown as percentage of input control (% Inp). IgG serves as a CHIP control. (e) Relative expression of HDAC1 in CD4+ T cells transduced and cultured as in d. Results are relative to the baseline expression of HDAC1 in unstimulated CD4+ T cells transduced with Lenti-CTR, set as 1. Error bars represent s.d. Student’s t test. **P<0.01. NS, not significant. All data are from three independent experiments (mean±s.d. of technical triplicates).
Collectively, our data suggest that lnc-Smad3 recruits HDAC1 to Smad3 promoter region and suppresses Smad3 transcription, while TGF-β-induced Smad3 reduces lnc-Smad3 expression via accumulating at lnc-Smad3 promoter regions. TGF-β-mediated reduction of lnc-Smad3 relieves its suppression on Smad3 promoter region, and hence allows the accumulation of Ash1l at Smad3 promoter regions to induce its H3K4me3 modification. Increased induction of Smad3 by Ash1l subsequently facilitates Foxp3 expression, contributing to the polarization of Treg cells and prevention of autoimmunity (Supplementary Fig. 12).
Discussion
H3K4 histone methylation is one of the most characterized epigenetic modifications, and some associated enzymes have emerged to be critical regulators of cell development especially in hematopoiesis23,24,25. H3K4 methyltransferase Smyd3 has been shown to target Foxp3 promoter region to increase its expression, promote iTreg cell development and protect mice from exacerbated inflammation during respiratory syncytial virus infection by a TGF-β-Smad3-dependent mechanism26. However, the functions of other H3K4 histone modifying enzymes in Treg cell differentiation remain poorly understood. In previous work, we have identified Ash1l, one of the H3K4 methyltransferases, as a negative regulator of innate inflammatory immune response13. Here, we demonstrate that Ash1l enhances TGF-β-induced Smad3 and Foxp3 expression and subsequently promotes iTreg cell polarization. Our data identify a new epigenetic modifier in Treg cell development and expand the function of Ash1l in immunity. Furthermore, ASH1L, SMAD3 and FOXP3 are all downregulated in CD4+ T cells from PBMCs of patients with rheumatoid arthritis, indicating the potential significance of ASH1L/SMAD3/FOXP3 pathway in human autoimmune pathogenesis.
Notably, whether the human Foxp3+ T cells induced by TGF-β stimulation in vitro could physiologically represent in vivo functional Treg cells are still controversial. Although TGF-β could induce Foxp3 expression both in human and mouse CD4+ T cells, the induced human Foxp3+ cells are functionally heterogeneous, with non-regulatory subpopulations showing little in vitro suppressive activity and even secrete proinflammatory cytokines such as IFN-γ27. Highly specific Treg cell surface markers in addition to Foxp3 are required to identify and purify the regulatory subpopulations in humans. The feasibility of using iTreg cells as a therapeutic intervention in human diseases is also limited by the stability of infused Treg cells27. Considering the possible plasticity of Treg cells towards other pathogenic T cell subsets such as Th17 cells, it is necessary to confirm the purity and stability of Treg cells during Treg cell-based therapy in humans. In addition, the detailed molecular mechanisms of Treg cell-mediated suppression in humans still remain to be determined. Altogether, it requires further investigation to clarify the function of Ash1l in human Treg cell development and human inflammatory immune diseases, especially via the improved methods for induction of immunosuppressive, purified and stable human iTreg cells.
Interestingly, unlike Smyd3, we find that Ash1l selectively targets Smad3 promoter region but not that of Foxp3. Ash1l promotes Foxp3 expression indirectly via enhancing the expression of its upstream molecule Smad3. The TGF-β-induced Smad3 acts as an important transcriptional factor to promote Foxp3 expression6,7. Vitamin A metabolite retinoic acid can enhance the expression and phosphorylation of Smad3, subsequently leading to an increase in Foxp3 expression28. The β-galactoside-binding protein galectin-9 acts synergistically with TGF-β signalling to enforce iTreg cell differentiation and maintenance via forming a positive feedback loop with Smad3 (ref. 29). TGF-β stimulation in T cells can induce Smad3 activation by phosphorylation, however, the epigenetic mechanisms governing TGF-β-dependent Smad3 expression is still unclear. By identifying the chromatin modifier Ash1l as a positive regulator of Smad3, we expand the epigenetic regulation network of TGF-β-Smad signalling and iTreg cell polarization.
lncRNAs have emerged as key regulators in multiple biological processes such as immune response, hematopoiesis, oncogenesis and so on30,31,32,33. We previously showed lnc-DC is selectively expressed in human dendritic cells and is required for the maturation and activation of dendritic cells34. Some T helper cell-specific lncRNAs have been identified and shown to regulate T cell differentiation. For example, linc-MAF-4 suppresses the Th2 polarization by associating with the chromatin modifiers LSD1 and Ezh2 (ref. 19). Another lncRNA, lincR-Ccr2-5′AS is identified as a Th2-specific lncRNA, which enhances the expression of numerous Th2-associated genes and promotes Th2 migration to lung tissue20. Rmrp, an lncRNA associated with human cartilage-hair hypoplasia, was shown to regulate the transcriptional expression of RORγt target genes, hence controlling the differentiation of Th17 cells and development of inflammatory diseases35. However, until now, lncRNAs that specifically regulate Treg cell polarization are still unknown. Here, we identified an lncRNA, named as lnc-Smad3, whose expression is inversely correlated with that of Smad3, is involved in suppression of iTreg cell induction via inhibiting TGF-β-mediated Smad3 expression.
Various lncRNAs act as modular scaffold or recruiter of histone modification complexes36,37. In our study, we found Lnc-Smad3 is involved in kept of the compact chromatin structure of the Smad3 promoter by interacting with HDAC1. The downregulation of lnc-Smad3 induced by TGF-β signalling hence allows Ash1l to be recruited and then activate the transcription of Smad3. Thus, during TGF-β-mediated iTreg cell polarization, lnc-Smad3 does not act as a scaffold or recruiter of Ash1l but functions as a guard of Smad3 promoter region via preventing the accumulation of Ash1l. Lnc-Smad3 regulates the chromatin state of Smad3 promoter mainly in the nucleus. However, a small portion of lnc-Smad3 is also detected in the cytoplasm. The biological function of cytoplasmic lnc-Smad3 awaits further investigations. It’s also intriguing to find out whether additional lncRNAs or chromatin modifiers might directly interact with Ash1l and recruit it specifically to Smad3 promoter region.
Taken together, our research identifies Ash1l and lnc-Smad3 as two opposing critical epigenetic modifiers that regulate TGF-β-mediated Treg cell polarization. We provide new insights into the epigenetic modifications of Treg cell polarization and immune homoeostasis and suggest possible clues for intervention of systemic inflammation and autoimmune diseases.
Methods
Mice
Ash1l-silenced mice on FVB background were from Dr Tian Xu (Institute of Developmental Biology and Molecular Medicine, Fudan University), and were backcrossed (12 polarizations) onto the C57BL/6J background13. Rag1−/− mice on the C57BL/6 background were from Shanghai Laboratory Animal Center, Chinese Academy of Science. Smad3-KO mice were kindly provided by Dr Xiao Yang (Laboratory Animal Center, Academy of Military Medical Science); CD45.1+ mice were from The Jackson Laboratory (B6.SJL-Ptprca Pepcb/BoyJ; 002014). All mice were maintained under specific pathogen-free conditions and used at 6–8 weeks of age. All animal experiments were approved by the Scientific Investigation Board of Second Military Medical University, Shanghai.
Human subjects
A total of 10 rheumatoid arthritis (RA) and 9 healthy control (HC, with no history of autoimmune disease) patients were recruited from Peking Union Medical College Hospital and Peking University People’s Hospital and their peripheral blood samples were prepared from patients after informed consent was provided38. RA was diagnosed according to the criteria of the American College of Rheumatology. The clinical characteristics of the individuals are shown in Supplementary Table 1. The study was approved by the Research Ethics Boards of Peking Union Medical College Hospital and Peking University People’s Hospital.
Reagents
Anti-mouse CD11c, CD8 and B220-coated magnetic beads were from Miltenyi Biotech. Recombinant mouse GM-CSF and IL-4 were from Peprotech EC. APC-conjugated anti-mouse Foxp3 (FJK-16s), GITR (DTA-1), IFN-γ (XMG1.2), CTLA-4 (UC10-4B9), FITC-conjugated anti-mouse CD8a (53-6.7), IL-4 (BVD6-24G2), IL-10 (JES5-16E3), and anti-IFN-γ (R4-6A2), anti-IL-4 (11B11) mAbs were from eBioscience. FITC-conjugated anti-mouse Foxp3 (MF23), CD25 (7D4), PE-Cy7-conjugated anti-mouse CD4 (RM4-5), APC-conjugated anti-mouse CD62L (MEL-14), and anti-CD3 (145-2C11), anti-CD28 (37-51) mAbs, Golgi Plug were from BD PharMingen. FITC-conjugated anti-mouse CD44 (IM7), APC-conjugated IL-17A (TC11-18H10), recombinant mouse IL-2, IL-6 and IL-12 were from BioLegend. Recombinant human TGF-β was from R&D Systems. Antibody specific to Ash1l (sc-98301X) was from Santa Cruz. Antibody specific to HDAC1 (ab7028) was from Abcam. The antibodies for cell staining were used at recommended dilution rates (1:1,000) according to the manufacturers’ instructions. TRIzol reagents were from Invitrogen. PrimeScript RT-PCR Kit (2641A) and SYBR Premix ExTaq Kit (RR420) were from Takara Bio Inc. RPMI1640 medium and FBS (fetal bovine serum) were from PAA laboratories.
Human PBMC isolation and CD4+ T cells sorting
Human PBMCs were collected and purified by Ficoll-Paque PLUS (GE healthcare) density gradient centrifugation. In brief, collected whole-blood samples were diluted and mixed with Hanks' balanced salt solution (HBSS). Then blood mixture was slowly added to layer over Ficoll. PBMCs were collected at the interface of the upper layer and the Ficoll after centrifugation (400g, 30 min). CD4+ T cells were sorted from fresh PBMCs with PerCP-conjugated anti-human CD4 antibody (Biolegend, 317432) by FACS Aria II flow cytometer (BD Biosciences). The FACS sorting strategy was introduced in Supplementary Fig. 13.
T cell isolation and polarization
Naive CD4+ T cells were purified from splenocytes, mLN (mesenteric lymph nodes) or thymus with mouse naive CD4+ T cell isolation kit (Stemcell, 19765) and cultured under neutral (Th0) conditions with anti-CD3 (1 μg ml−1), soluble anti-CD28 mAbs (1 μg ml−1) and 10% (vol/vol) FBS in RPMI1640, unless otherwise indicated. For T cell polarization, CD4+ T cells were cultured in the neutral medium described above and stimulated with polarizing cytokines as follows: for iTreg, anti-IFN-γ (10 μg ml−1), anti-IL-4 (10 μg ml−1), IL-2 (5 ng ml−1) and recombinant human TGF-β (10 ng ml−1); for Th1, anti-IL-4 (10 μg ml−1), IL-2 (5 ng ml−1) and IL-12 (10 ng ml−1); and for Th17, anti-IFN-γ (10 μg ml−1), anti-IL-4 (10 μg ml−1), human IL-6 (10 ng ml−1) and recombinant human TGF-β (1 ng ml−1). Differentiated iTreg cells were further enriched with mouse CD25 positive selection kit (Stemcell, 18761).
In vitro suppression assay
A total of 5 × 104 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled naive CD4+ effector T cells from CD45.1 mice were cultured with 1 × 104 CD11c+ DC used as antigen-presenting cells in 96-well round-bottom plates. Overall, 2.5 × 104 differentiated and purified WT or Ash1l-silenced iTreg cells were added as suppressive cells. Cells were stimulated with anti-CD3 (1 μg ml−1) for 3 days. T cell proliferation was determined by CFSE dilution with flow cytometry.
Induction of colitis
Mice were presensitizated with 150 μl of 1% (w/v) TNBS solution diluted in acetone/olive oil (Sigma-Aldrich, MO, USA) on the back. On day 7, mice were further given 100 μl of 2.5% (w/v) TNBS solution (diluted in 50% ethanol) or ethanol only intrarectally through a catheter inserted into the colon 4 cm proximal to the anus. Beginning from the second sensitization, inflammatory bowel disease development was monitored and recorded by measuring the body weight daily. Mice were killed 3 days after the second sensitization and subjected to examination of colon morphology and histology, as well as Treg cells proportion. T cell adoptive transfer colitis was performed as follows. Rag1−/− mice were injected intravenously with 4 × 105 CD4+CD25− cells from CD45.1 WT mice with or without 1 × 105 WT or Ash1l-silenced CD4+CD25+ differentiated iTreg cells. Weight changes were monitored weekly. Mice were killed at week 6 and subjected to examination of colon morphology and histology.
In vivo iTreg induction
Rag1−/− mice were injected intravenously with 4 × 105 CD4+CD25− cells from WT or Ash1l-silenced mice. Monitored the weight changes and scarified the mice at week 4. T cells were isolated and Foxp3 expression was analysed with flow cytometry.
Flow cytometry
For cell surface staining, the single-cell suspensions were incubated with the antibody cocktails for 20 min at 4 °C. For intracellular staining, cells were fixed and permeabilized with 4% paraformaldehyde for 20 min at 4 °C followed by intracellular staining with specific anti-cytokine antibodies. Data were obtained on an LSR II and analysed with FACSDiva software (both from BD Biosciences). The FACS gating strategies were represented in Supplementary Fig. 13.
Quantitative real time PCR assay
SYBR RT-PCR kit (Takara) and LightCycler (Roche) were used for quantitative RT-PCR analysis39. Data were normalized to β-actin expression. Sequences of the primers for quantitative real time RT-PCR are in Supplementary Table 2.
Nucleus and cytoplasm fraction isolation
Cells were lysed in 0.1% NP40 ice-cold PBS with protease inhibitor cocktail (Calbiochem, La Jolla, CA, USA) and Ribonucleoside Vanadyl Complex (10 mM) (New England BioLabs), then after short centrifugation, the supernatant was collected as cytoplasmic fraction and the remainder with additional washing were considered as nuclear pellets.
Smad2/3 ELISA assay
The activated Smad2/3 in nuclear extract or the whole-cell lysate was detected with Smad2/3 ELISA Kit according to the manufacture’s protocol (Signosis, CA, USA, TE-0011). In brief, 5 μg nuclear extract or the whole-cell lysate was added in the 96-well clear plate pre-immobilized with the Smad2/3 consensus sequencing oligo, incubated and washed. Then the activated Smad2/3 binding to the oligo was detected with a specific antibody against Smad2/3 subunit and a HRP-conjugated secondary antibody. The optical density of each well was determined with a microplate reader at 450 nm.
Plasmid constructions
Recombinant vectors encoding mouse Smad3 (NM_016769.4) were constructed by PCR-based amplification from cDNA of mouse CD4+ T cells and then subcloned into the pcDNA3.1 eukaryotic expression vector (Invitrogen). Lnc-Smad3, Ash1l-fragment 1, 2, 3, and Ash1l-fragment3 mutant expression vectors were constructed by PCR-based amplification from cDNA of mouse CD4+ T cells and then subcloned into pCDH cDNA Cloning and Expression Lentivectors (System Biosciences). All constructs were confirmed by DNA sequencing. These plasmids transfected into HEK293T cell line (from American Type Culture Collection) with JetPEI reagents (Polyplus), and the protein coding capacity was tested by western blotting assay. The Smad3 promoter luciferase reporter was constructed via cloning the fragment of the Smad3 promoter (1,000 bp upstream of transcription start site) into the pGL3 luciferase-reporter vector (Promega) and the DNA sequence of the insert was verified.
Reporter assay
HepG2 cells (3 × 104 cells per well, 96-well plate, from American Type Culture Collection) were transiently transfected with the lnc-Smad3 expression vector, or empty control vector as well as the Smad3 promoter firefly luciferase-reporter construct and Renilla luciferase-reporter vector (Promega) with Fugene HD (Promega). Cells were stimulated with TGF-β 24 h after transfection. Twenty-four hours after stimulation luciferase expression was determined by measuring luminescence with the Dual-Luciferase Reporter Assay System (Promega). The firefly luciferase activity was normalized to renilla luciferase activity.
Knockdown and overexpression
Naive CD4+ T cells were stimulated with plate-coated anti-CD3 (1 μg ml−1), soluble anti-CD28 mAbs (1 μg ml−1) and IL-2 (10 ng ml−1) for 24 h before knockdown or overexpression assay. For knockdown assay, the target sequence of lnc-Smad3 was 5′-CATTGGACCATTTGATTCTTCCTAA-3′ (bolded and underlined in the following lnc-Smad3 sequence); the control siRNA sequence was 5′-CATCCAGTTTATTAGCTTCCGTTAA-3′. siRNA was transfected into activated CD4+ naive T cells through the use of Lipofectamine 2000 according to the manufacture’s protocol (Life Technologies). For overexpression experiments, virus solutions containing lentivirus and polybrene (8 μg ml−1) were added to the activated CD4+ T cells, followed by centrifugation at 2,500 r.p.m for 2 h at room temperature. Virus supernatants were replaced with fresh culture medium at 6 h after infection. Cells were cultured for an additional 18 h and then sorted as enhanced green fluorescent protein (EGFP)-positive cells. Sorted cells were cultured for 3 days under iTreg cell conditions. Transduction efficiency was determined by EGFP expression and knockdown efficiency was measured by RT-PCR.
>mouse lnc-Smad3 transcript (GenBank Accession number: KY652933) CGGCGCGTGCGCACAGGACGGGACGGGAGGGCGGAGCGACTGCGCAGATCAGGAAAATTGTCACCTCTGGCCTCAGGGAAACTGAGGCTCTGAGCAGTTAAGAGGCCAACGATCCAGGTTTATGCTATCAGTGTCTGAGATACAATTAAGTCACCTTTTTGGGTGACATTTCCCTTGACTAGTACCTCAAGATTATGATCCAGGTGGACCATCCCTCCATTGCCTCCAGACATGTCTATGACGCTACCTAGGGATTGTGAAGATTTACCACCTGGTGGAAAATTAAAAAAAAAGACTTATTCTGCAAATTGACCAAGCTTAAGAGATACAGCACTGAGAATTCACCCACTACAGAGCTGGTAACCAGGCCTTCAGAGTAAGCTGTGATGTATAGCCATTCTCCTCAGCAGCCTGTTTGACTGAGGGATATAGGAATGACTGCCCTTACCCATGTTTTTGTTCAGATTTATGGTTCTAAATCTGATGGAAAATCTATCATTGGACCATTTGATTCTTCCTAAAGAAAGGCCAAACCACCTGTCAAACCCACGAGCAACACAGCAATGGCTAAGCTGAAGAAGCCAAGTTATATGTCTAGCTCCTCTGTGGGTGGCAGGATCCAGCTGTGGGGAGTCCAAGGTTCCTTGTGTTCTTCTGGCTTGACTATGATATTTCCAATTTGGAGGCACAAAGAATTGAGAACTTGGGGATCCACTGAGGTCTGAGGCATGGTGGTACCAGAAGAAATAAAGGGGCCATCAGCATGAAGCTGGTCTCCCATTGACAGCACCCTGACTTCTCCATCAGGTTCTATCAGCATGTCTACTGTGAGGTTGGTGACGCTGTCTGCAGGTGGGTATGCTTCAATCACGCCACCTGGTCAGGAAACCATGGAGTAGAGGAAAAGACACAGTCAAGGGAATGCTATGCTTATAGCAGGAGGAAATGCTCTGTGTTTTAGCAAGGGAACTTAGACTATCTCACACCTGTCATATCCTGGGAAACTTTGGGTGAGTAGTTCAACCTTTGGCCTCCTTTTCCTTATTTATAGAATGGAGGCATAATATCTACCTTGCCAAGCTTTTGTGAAGTTTGAGAAACTGAGTCTATGGTATTTAGCCTGGTACTTGGAACATGGGAGGGCTCAATGAACAGTAAGTAGTCTTTATTATGATCATAATTACAAATATTACAAATACTTTATAAAAGCCATACTTAGAACAACTCATTACGTTAATAAAGAATCCAAGGATTGTGGTC
Chromatin immunoprecipitation
CHIP analyses were performed with the following antibodies: RNA Polymerase II (Pol II), acetyl-Histone H3 (Lys27), trimethyl-Histone H3 (Lys4), Ash1l and HDAC1. Fold enrichment was quantified using quantitative RT-PCR and calculated as a percentage of Input chromatin (% input). Sequences of the primers for amplification of the Smad2, Smad3, Smad4, Foxp3 and lnc-Smad3 promoter regions are in Supplementary Table 3.
Chromatin accessibility analysis
For chromatin accessibility analysis, nucleus were pretreated with DNase I (0.1 U l−1, Promega) at 37 °C for 30 min and then stopped by EDTA (50 mM). Genome DNA was extracted and subjected to quantitative RT-PCR. Data were presented as changed fold concluded with 2ΔCt, with relative to CD4+ T cells transduced with Lenti-CTR, set as 1.
Rapid amplification of cloned cDNA ends
Total RNA extracted from mouse B220+ B cells was subjected to RACR PCR with SMARTer RACE 5′/3′ Kit according to the manufacture’s protocol (Clontech, CA, USA, 634558).
RNA immunoprecipitation
RNA immunoprecipitation (RIP) was performed with antibodies specific to mouse Ash1l, Smad3 and HDAC1 by using Magna RIP RNA-Binding Protein Immunoprecipitation Kit according to the manufacture’s protocol (Millipore, CA, USA, 17-700).
RNA fluorescence in situ hybridization
Fluorescence-conjugated lnc-Smad3 probes were used for FISH assay. Naive CD4+T cells were fixed in 4% formaldehyde plus 10% acetic acid in PBS for 15 min at room temperature, and then were permeabilized in PBS plus 0.2%–0.5% Triton X-100 and 5 mM vanadyl ribonucleoside complex (10 mM) (New England BioLabs) for 5 min on ice, washed in PBS three times and rinsed once in 2 × SSC buffer34. Hybridization was carried out using DNA probe sets according to the protocol of Biosearch Technologies. Cells were observed with a Leica TCS SP2 confocal laser microscopy.
Statistical analysis
The statistical significance between two groups was determined by Student’s t test. P values of <0.05 were considered statistically significant (*P<0.05, **P<0.01).
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author on reasonable request.
Additional information
How to cite this article: Xia, M. et al. Ash1l and lnc-Smad3 coordinate Smad3 locus accessibility to modulate iTreg polarization and T cell autoimmunity. Nat. Commun. 8, 15818 doi: 10.1038/ncomms15818 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Ohkura, N., Kitagawa, Y. & Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013).
Goldstein, J. D. et al. Role of cytokines in thymus versus peripherally derived-regulatory T cell differentiation and function. Front. Immunol. 4, 155 (2013).
Chen, W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Zhou, L. et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240 (2008).
Gu, A. D., Wang, Y., Lin, L., Zhang, S. S. & Wan, Y. Y. Requirements of transcription factor Smad-dependent and -independent TGF-beta signaling to control discrete T-cell functions. Proc. Natl Acad. Sci. USA 109, 905–910 (2012).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9, 194–202 (2008).
Kanno, Y., Vahedi, G., Hirahara, K., Singleton, K. & O'Shea, J. J. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 30, 707–731 (2012).
Toker, A. & Huehn, J. To be or not to be a Treg cell: lineage decisions controlled by epigenetic mechanisms. Sci. Signal. 4, pe4 (2011).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012).
Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. & Cavalli, G. Genome regulation by polycomb and trithorax proteins. Cell 128, 735–745 (2007).
Xia, M. et al. Histone methyltransferase Ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme A20. Immunity 39, 470–481 (2013).
Tanaka, Y., Nakayama, Y., Taniguchi, M. & Kioussis, D. Regulation of early T cell development by the PHD finger of histone lysine methyltransferase ASH1. Biochem. Biophys. Res. Commun. 365, 589–594 (2008).
Fang, K., Zhang, S., Glawe, J., Grisham, M. B. & Kevil, C. G. Temporal genome expression profile analysis during T-cell-mediated colitis: identification of novel targets and pathways. Inflamm. Bowel Dis. 18, 1411–1423 (2012).
Noble, C. L. et al. Regional variation in gene expression in the healthy colon is dysregulated in ulcerative colitis. Gut 57, 1398–1405 (2008).
Zucchelli, S. et al. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 22, 385–396 (2005).
Roychoudhuri, R. et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature 498, 506–510 (2013).
Ranzani, V. et al. The long intergenic noncoding RNA landscape of human lymphocytes highlights the regulation of T cell differentiation by linc-MAF-4. Nat. Immunol. 16, 318–325 (2015).
Hu, G. et al. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nat. Immunol. 14, 1190–1198 (2013).
Yu, J. et al. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 24, 575–590 (2006).
Tang, Y. N. et al. Epigenetic regulation of Smad2 and Smad3 by profilin-2 promotes lung cancer growth and metastasis. Nat. Commun. 6, 8230 (2015).
Tusi, B. K. et al. Setd1a regulates progenitor B-cell-to-precursor B-cell development through histone H3 lysine 4 trimethylation and Ig heavy-chain rearrangement. FASEB J. 29, 1505–1515 (2015).
Ortega-Molina, A. et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat. Med. 21, 1199–1208 (2015).
Mishra, B. P. et al. The histone methyltransferase activity of MLL1 is dispensable for hematopoiesis and leukemogenesis. Cell Rep. 7, 1239–1247 (2014).
Nagata, D. E. et al. Epigenetic control of Foxp3 by SMYD3 H3K4 histone methyltransferase controls iTreg development and regulates pathogenic T-cell responses during pulmonary viral infection. Mucosal Immunol. 8, 1131–1143 (2015).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Xiao, S. et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 181, 2277–2284 (2008).
Wu, C. et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41, 270–282 (2014).
Turner, M., Galloway, A. & Vigorito, E. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat. Immunol. 15, 484–491 (2014).
Carpenter, S. et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792 (2013).
Luo, M. et al. Long non-coding RNAs control hematopoietic stem cell function. Cell Stem Cell 16, 426–438 (2015).
Arab, K. et al. Long noncoding RNA TARID directs demethylation and activation of the tumor suppressor TCF21 via GADD45A. Mol. Cell 55, 604–614 (2014).
Wang, P. et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344, 310–313 (2014).
Huang, W. et al. DDX5 and its associated lncRNA Rmrp modulate TH17 cell effector functions. Nature 528, 517–522 (2015).
Tsai, M. C. et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010).
Xing, Z. et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell 159, 1110–1125 (2014).
He, J. et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 39, 770–781 (2013).
Yang, M. et al. K33-linked polyubiquitination of Zap70 by Nrdp1 controls CD8(+) T cell activation. Nat. Immunol. 16, 1253–1262 (2015).
Acknowledgements
We would like to thank Ms. Ling Ma, Dr Pin Wang and Dr Sheng Xu for the technical assistance; Dr Binfeng Lu (University of Pittsburgh) and Dr Jingde Zhu (Cancer Epigenetics Laboratory, Anhui Cancer Hospital) for helpful discussions. This work was supported by grants from the National Key Basic Research Program of China (2013CB530503), China Postdoctoral Science Foundation (2015M570052), National Natural Science Foundation of China (31400777, 31390431, 31370864, 81671546) and CAMS Innovation Fund for Medical Sciences (2016-12M-1-003).
Author information
Authors and Affiliations
Contributions
X.C. designed and supervised the research; M.X., J.L., S.L., K.C., H.L., M.J., X.X., Y.X., W.L., Y.G., X.Z., Z.L. and L.Y. performed experiments; Y.Q. provided reagents; C.Z., R.L., X.Z. and Z.L. provided clinical samples; X.C., M.X., J.L. and S.L. analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xia, M., Liu, J., Liu, S. et al. Ash1l and lnc-Smad3 coordinate Smad3 locus accessibility to modulate iTreg polarization and T cell autoimmunity. Nat Commun 8, 15818 (2017). https://doi.org/10.1038/ncomms15818
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms15818
This article is cited by
-
The histone methyltransferase ASH1L protects against bone loss by inhibiting osteoclastogenesis
Cell Death & Differentiation (2024)
-
Upregulated lncARAT in Schwann cells promotes axonal regeneration by recruiting and activating proregenerative macrophages
Molecular Medicine (2022)
-
LncRNA-mediated DNA methylation: an emerging mechanism in cancer and beyond
Journal of Experimental & Clinical Cancer Research (2022)
-
Distinct clinical phenotypes for Crohn’s disease derived from patient surveys
BMC Gastroenterology (2021)
-
Long noncoding RNA: a dazzling dancer in tumor immune microenvironment
Journal of Experimental & Clinical Cancer Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.