Abstract
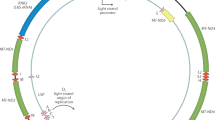
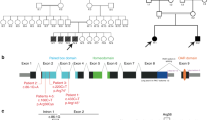
Experimental evidence for human mitochondrial DNA (mtDNA) recombination was recently obtained in an individual with paternal inheritance of mtDNA1 and in an in vitro cell culture system2. Whether mtDNA recombination is a common event in humans remained to be determined. To detect mtDNA recombination in human skeletal muscle, we analyzed the distribution of alleles in individuals with multiple mtDNA heteroplasmy using single-cell PCR and allele-specific PCR. In all ten individuals who carried a heteroplasmic D-loop mutation and a distantly located tRNA point mutation or a large deletion, we observed a mixture of four allelic combinations (tetraplasmy), a hallmark of recombination. Twelve of 14 individuals with closely located heteroplasmic D-loop mutation pairs contained a mixture of only three types of mitochondrial genomes (triplasmy), consistent with the absence of recombination between adjacent markers. These findings indicate that mtDNA recombination is common in human skeletal muscle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kraytsberg, Y. et al. Recombination of human mitochondrial DNA. Science 304, 981 (2004).
D'Aurelio, M. et al. Heterologous mitochondrial DNA recombination in human cells. Hum. Mol. Genet. 13, 3171–3179 (2004).
Rokas, A., Ladoukakis, E. & Zouros, E. Animal mitochondrial DNA recombination revisited. Trends Ecol. Evol. 18, 411–417 (2003).
Awadalla, P., Eyre-Walker, A. & Smith, J.M. Linkage disequilibrium and recombination in hominid mitochondrial DNA. Science 286, 2524–2525 (1999).
Arctander, P. Mitochondrial recombination? Science 284, 2090–2091 (1999).
Merriweather, D.A. & Kaestle, F.A. Mitochondrial recombination? (continued). Science 285, 837 (1999).
Kivisild, T. & Villems, R. Questioning evidence for recombination in human mitochondrial DNA. Science 288, 1931 (2000).
Jorde, L.B. & Bamshad, M. Questioning evidence for recombination in human mitochondrial DNA. Science 288, 1931 (2000).
Kumar, S., Hedrick, P., Dowling, T. & Stoneking, M. Questioning evidence for recombination in human mitochondrial DNA. Science 288, 1931 (2000).
Parsons, T.J. & Irwin, J.A. Questioning evidence for recombination in human mitochondrial DNA. Science 288, 1931 (2000).
Elson, J.L. et al. Analysis of European mtDNAs for recombination. Am. J. Hum. Genet. 68, 145–153 (2001).
Herrnstadt, C. et al. Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am. J. Hum. Genet. 70, 1152–1171 (2002).
Smith, J.M. & Smith, N.H. Recombination in animal mitochondrial DNA. Mol. Biol. Evol. 19, 2330–2332 (2002).
Hagelberg, E. Recombination or mutation rate heterogeneity? Implications for Mitochondrial Eve. Trends Genet. 19, 84–90 (2003).
Sato, A. et al. Rare creation of recombinant mtDNA haplotypes in mammalian tissues. Proc. Natl. Acad. Sci. USA 102, 6057–6062 (2005).
Schwartz, M. & Vissing, J.N. Paternal inheritance of mitochondrial DNA. N. Engl. J. Med. 347, 576–580 (2002).
Filosto, M. et al. Lack of paternal inheritance of muscle mitochondrial DNA in sporadic mitochondrial myopathies. Ann. Neurol. 54, 524–526 (2003).
Schwartz, M. & Vissing, J. No evidence for paternal inheritance of mtDNA in patients with sporadic mtDNA mutations. J. Neurol. Sci. 218, 99–101 (2004).
Coller, H.A. et al. High frequency of homoplasmic mitochondrial DNA mutations in human tumors can be explained without selection. Nat. Genet. 28, 147–150 (2001).
Pääbo, S., Irwin, D.M. & Wilson, A.C. DNA damage promotes jumping between templates during enzymatic amplification. J. Biol. Chem. 265, 4718–4721 (1990).
Nekhaeva, E. et al. Clonally expanded mtDNA point mutations are abundant in individual cells of human tissues. Proc. Natl. Acad. Sci. USA 99, 5521–5526 (2002).
Khrapko, K., Nekhaeva, E., Kraytsberg, Y. & Kunz, W. Clonal expansions of mitochondrial genomes: implications for in vivo mutational spectra. Mutat. Res. 522, 13–19 (2003).
Ohno, K. et al. MELAS- and Kearns-Sayre-type co-mutation with myopathy and autoimmune polyendocrinopathy. Ann. Neurol. 39, 761–766 (1996).
Bidooki, S.K., Johnson, M.A., Chrzanowska-Lightowlers, Z., Bindoff, L.A. & Lightowlers, R.N. Intracellular mitochondrial triplasmy in a patient with two heteroplasmic base changes. Am. J. Hum. Genet. 60, 1430–1438 (1997).
Zsurka, G. et al. Tissue dependent co-segregation of the novel pathogenic G12276A mitochondrial tRNALeu(CUN) mutation with the A185G D-loop polymorphism. J. Med. Genet. 41, e124 (2004).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
Acknowledgements
We thank A. de Grey for his comments on the manuscript and U. Strube for technical assistance. This study was supported by research grants of the Deutsche Forschungsgemeinschaft and the Bundesministerium für Bildung und Forschung to W.S.K. and by grants from the US National Institutes of Health to K.K.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic representation of positions of deletions, D-loop point mutations and used PCR primers. (PDF 64 kb)
Supplementary Fig. 2
Comparison of mtDNA heteroplasmy determined by SYBR Green staining and radioactive detection ('last cycle hot' PCR). (PDF 13 kb)
Supplementary Fig. 3
Purity of agarose gel electrophoresis-separated genomic DNA samples determined by multiplex PCR. (PDF 81 kb)
Supplementary Table 1
Comparison of D-loop mutant loads as determined in allele-specific PCR products and gel-separated DNA fractions. (PDF 23 kb)
Supplementary Table 2
Frequency of heteroplasmic D-loop mutations in post-mitotic tissues. (PDF 8 kb)
Supplementary Table 3
Overview of patients with multiple heteroplasmic mutations in the coding region and the D-loop. (PDF 8 kb)
Supplementary Table 4
Primer sequences and mutation detection. (PDF 15 kb)
Supplementary Note
Proof of the in vivo origin of allelic tetraplasmy. (PDF 10 kb)
Rights and permissions
About this article
Cite this article
Zsurka, G., Kraytsberg, Y., Kudina, T. et al. Recombination of mitochondrial DNA in skeletal muscle of individuals with multiple mitochondrial DNA heteroplasmy. Nat Genet 37, 873–877 (2005). https://doi.org/10.1038/ng1606
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1606
This article is cited by
-
Prevention of mitochondrial genomic instability in yeast by the mitochondrial recombinase Mhr1
Scientific Reports (2019)
-
Heteroplasmic mitochondrial DNA mutations in normal and tumour cells
Nature (2010)
-
Frequency and Pattern of Heteroplasmy in the Control Region of Human Mitochondrial DNA
Journal of Molecular Evolution (2008)
-
Recombination in Mitochondrial DNA of European Mussels Mytilus
Journal of Molecular Evolution (2008)
-
Mitochondrial DNA sequence variation and mutation rate in patients with CADASIL
Neurogenetics (2006)