Abstract
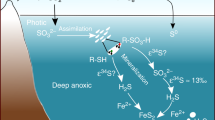
Iron formations deposited in marine settings during the Precambrian represent large sinks of iron and silica, and have been used to reconstruct environmental conditions at the time of their formation. However, the observed mineralogy in iron formations, which consists of iron oxides, silicates, carbonates and sulfides, is generally thought to have arisen from diagenesis of one or more mineral precursors. Ferric iron hydroxides and ferrous carbonates and silicates have been identified as prime candidates. Here we investigate the potential role of green rust, a ferrous–ferric hydroxy salt, in the genesis of iron formations. Our laboratory experiments show that green rust readily forms in early seawater-analogue solutions, as predicted by thermodynamic calculations, and that it ages into minerals observed in iron formations. Dynamic models of the iron cycle further indicate that green rust would have precipitated near the iron redoxcline, and it is expected that when the green rust sank it transformed into stable phases within the water column and sediments. We suggest, therefore, that the precipitation and transformation of green rust was a key process in the iron cycle, and that the interaction of green rust with various elements should be included in any consideration of Precambrian biogeochemical cycles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bekker, A. et al. Iron formation: the sedimentary product of a complex interplay among mantle, tectonic, and biospheric processes. Econ. Geol. 105, 467–508 (2010).
Klein, C. Some Precambrian banded-iron formations (BIFs) from around the world: their age, geologic setting, mineralogy, metamorphism, geochemistry, and origin. Am. Mineral. 90, 1473–1499 (2005).
Cloud, P. Paleoecological significance of the banded iron formation. Econ. Geol. 68, 1135–1143 (1973).
Walker, J. C. G. Suboxic diagenesis in banded iron formations. Nature 309, 340–342 (1984).
Fischer, W. W. & Knoll, A. H. An iron shuttle for deepwater silica in Late Archean and early Proterozoic iron formations. Geol. Soc. Am. Bull. 121, 222–235 (2008).
Kappler, A., Pasquero, C., Konhauser, K. O. & Newmanm, D. K. Deposition of banded iron formations by anoxygenic phototrophic Fe(II)-oxidizing bacteria. Geology 33, 865–868 (2005).
Cairns-Smith, A. G. Precambrian solution photochemistry, inverse segregation, and banded iron formations. Nature 276, 807–808 (1978).
Rasmussen, B., Krapež, B. & Meier, D. B. Replacement origin for hematite in 2.5 Ga banded iron formation: evidence for postdepositional oxidation of iron-bearing minerals. Geol. Soc. Am. Bull. 126, 438–446 (2014).
Rasmussen, B., Krapež, B. & Muhling, J. R. Hematite replacement of iron-bearing precursor sediments in the 3.46-b. y.-old Marble Bar Chert, Pilbara craton, Australia. Geol. Soc. Am. Bull. 126, 1245–1258 (2014).
Trolard, F., Bourrié, G., Abdelmoula, M., Refait, P. & Feder, F. Fougerite, a new mineral of the pyroaurite-iowaite group: description and crystal structure. Clays Clay Miner. 3, 323–334 (2007).
Refait, P., Memet, J.-B., Bon, C., Sabot, R. & Génin, J.-M. R. Formation of the Fe(II)-Fe(III) hydroxysulfate green rust during marine corrosion of steel. Corros. Sci. 45, 833–845 (2003).
Legrand, L., Mazerolles, L. & Chaussé, A. Oxidation of carbonate green rust into ferric phases: solid-state reaction or transformation via solution. Geochim. Cosmochim. Acta 68, 3497–3507 (2004).
Génin, J.-M. R., Ruby, C., Géhin, A. & Refait, P. Synthesis of green rusts by oxidation of Fe(OH)2, their products of oxidation and reduction of ferric oxyhydroxides: Eh-pH Pourbaix diagrams. C. R. Geosci. 338, 433–446 (2006).
Zegeye, A. et al. Green rust formation controls nutrient availability in a ferruginous water column. Geology 40, 599–602 (2012).
Wiesli, R. A., Beard, B. L. & Johnson, C. M. Experimental determination and Fe isotope fractionation between Fe(II), siderite and “green rust” in abiotic systems. Chem. Geol. 211, 343–362 (2004).
Halevy, I. & Schrag, D. P. Sulfur dioxide inhibits calcium carbonate precipitation: implications for Mars and Earth. Geophys. Res. Lett. 36, L23201 (2009).
Beukes, N. J., Klein, C., Kaufman, A. J. & Hayes, J. M. Carbonate petrography, kerogen distribution, and carbon and oxygen isotope variations in an early Proterozoic transition from limestone to iron formation deposition, transvaal supergroup, South Africa. Bull. Soc. Econ. Geol. 85, 663–690 (1990).
Tosca, N. J., Guggenheim, S. & Pufahl, P. K. An authigenic origin for Precambrian greenalite: implications for iron formation and the chemistry of ancient seawater. Geol. Soc. Am. Bull. 128, 511–530 (2015).
Bruno, J., Wersin, P. & Stumm, W. On the influence of carbonate in mineral dissolution: II. The solubility of FeCO3 (s) at 25 °C and 1 atm total pressure. Geochim. Cosmochim. Acta 56, 1149–1155 (1992).
Walker, J. C. G., Hays, P. B. & Kasting, J. F. A negative feedback mechanism for the long-term stabilization of Earth’s surface temperature. J. Geophys. Res. 86, 9776–9872 (1981).
Grotzinger, J. P. & Kasting, J. F. New constraints on Precambrian ocean composition. J. Geol. 101, 235–243 (1993).
Ona-Nguema, G. et al. Iron(II, III) hydroxycarbonate green rust formation and stabilization from lepidocrocite bioreduction. Environ. Sci. Tech. 36, 16–20 (2002).
Tamaura, Y. Ferrite formation from the intermediate, green rust II, in the transformation of γ-FeO(OH) in aqueous suspension. Inorg. Chem. 24, 4363–4366 (1985).
Kwon, S.-K. et al. Influence of silicate ions on the formation of goethite from green rust in aqueous solution. Corros. Sci. 49, 2946–2961 (2007).
Hansen, C. R. H. & Poulsen, I. F. Interaction of synthetic sulphate “green rust” with phosphate and the crystallization of vivianite. Clays Clay Miner. 47, 312–318 (1999).
Bachan, A. & Kump, L. R. The rise of oxygen and siderite oxidation during the Lomagundi Event. Proc. Natl. Acad. Sci. USA 112, 6562–6567 (2015).
O’Loughlin, E. J., Kelly, S. D., Cook, R. E., Csencsits, R. & Kemner, K. M. Reduction of uranium(VI) by mixed iron(II)/Iron(III) hydroxide (green rust): formation of UO2 nanoparticles. Environ. Sci. Technol. 37, 721–727 (2003).
Loyaux-Lawniczak, S., Refait, P., Ehrhardt, J.-J., Lecomte, P. & Génin, J.-M. R. Trapping of Cr by the formation of ferrihydrite during the reduction of chromate ions by Fe(II)—Fe(III) hydroxysalt green rusts. Environ. Sci. Technol. 34, 438–443 (2000).
Refait, P., Drissi, H., Marie, Y. & Génin, J.-M. R. The substitution of Fe2+ ions by Ni2+ ions in green rust one compounds. Hyperfine Interact. 90, 389–394 (1994).
Rowe, C. D. & Wing, B. A. Physical sedimentology constrains the primary precipitates in banded iron formations. Goldschmidt abstract. 2701 (2015).
Bethke, C. Geochemical and Biogeochemical Reaction Modeling (Cambridge Press, 2008).
Johnson, J. W., Oelkers, E. H. & Hegelson, H. C. SUPCRT92: a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 °C. Comput. Geosci. 18, 899–947 (1992).
Tosca, N. J. et al. Geochemical modeling of evaporation processes on Mars: insight from the sedimentary record at Meridiani Planum. Earth Planet. Sci. Lett. 240, 122–148 (2005).
Rickard, D. & Luther, G. W. III. Chemistry of iron sulfides. Chem. Rev. 107, 514–562 (2007).
von Paris, P. et al. Warming the early Earth—CO2 reconsidered. Planet. Space Sci. 56, 1244–1259 (2008).
Crowe, S. A. et al. Sulfate was a trace constituent of Archean seawater. Science 346, 735–739.
Siever, R. The silica cycle in the Precambrian. Geochim. Cosmochim. Acta 65, 3265–3273 (1992).
Drissi, S. H., Refait, Ph., Abdelmoula, M. & Génin, J. M. R. The preparation and thermodynamic properties of Fe(II)-Fe(III) hydroxide-carbonate (green rust 1); Pourbaix diagram of iron in carbonate-containing aqueous media. Corros. Sci. 37, 2015–2041 (1995).
Haynes, W. M. CRC Handbook of Chemistry and Physics 97 edn (CRC Press, 2016).
Parker, V. B. & Khodakovskii, I. L. Thermodynamic properties of the aqueous ions (2 + and 3 +) of iron and the key compounds of iron. J. Phys. Chem. Ref. Data 24, 1699–1745 (1995).
Refait, Ph. & Génin, J. M. R. The transformation of chloride-containing green rust into sulphated green rust two by oxidation in mixed Cl− and SO42− aqueous media. Corros. Sci. 36, 55–65 (1994).
Olowe, A. A. & Génin, J. M. R. in Corrosion Science and Engineering: Proceedings of an International Symposium in honour of Marcel Pourbaix’s 85th Birthday Vol. 158 (eds Rapp, R. A., Gocken, N. A. & Pourbaix, A.) RT 297 (Rapports Techniques CEBELCOR, 1989).
Gayer, K. H. & Wootner, L. The hydrolysis of ferrous chloride at 25°. J. Am. Chem. Soc. 78, 3944–3946 (1956).
Downs, R. T. The RRUFF Project: An Integrated Study of the Chemistry, Crystallography, Raman and Infrared Spectroscopy of Minerals (19th General Meeting of the International Mineralogical Association, 2006).
Elderfield, H. & Schultz, A. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci. 24, 191–224 (1996).
Ganachaud, A. & Wunsch, C. Improved estimates of global ocean circulation, heat transport and mixing from hydrographic data. Nature 408, 453–457 (2000).
Millero, F. J., Sotolongo, S. & Izaguirre, M. The oxidation kinetics of Fe(II) in seawater. Geochim. Cosmochim. Acta 51, 793–801 (1987).
Druschel, G. K., Emerson, D., Sutka, R., Suchecki, P. & Luther, G. W. III Low-oxygen and chemical kinetic constraints on the geochemical niche of neutrophilic iron(II) oxidizing microorganisms. Geochim. Cosmochim. Acta 72, 3358–3370 (2008).
Anbar, A. D. & Holland, H. D. The photochemistry of manganese and the origin of banded iron formations. Geochim. Cosmochim. Acta 56, 2595–2603 (1992).
Hotinski, R. M., Kump, L. R. & Najjar, R. G. Opening Pandora’s Box: the impact of open system modeling on interpretations of anoxia. Paleoceanography 15, 267–279 (2000).
Sleep, N. H. & Zahnle, K. Carbon dioxide cycling and implications for climate on ancient Earth. J. Geophys. Res. 106, 1373–1399 (2001).
Kump, L. R. Jr & Seyfried, W. E. Hydrothermal Fe fluxes during the Precambrian: effect of low oceanic sulfate concentrations and low hydrostatic pressure on the composition of black smokers. Earth Planet. Sci. Lett. 235, 654–662 (2005).
Acknowledgements
We thank N. Tosca for valuable comments. I.H. acknowledges funding from a European Research Council Starting Grant 337183, and an Israeli Science Foundation Grant 764/12. I.H. is the incumbent of the Anna and Maurice Boukstein Career Development Chair at the Weizmann Institute of Science. Electron microscopy was carried out at the Moskowitz Center for Nano and Bio-Nano Imaging.
Author information
Authors and Affiliations
Contributions
I.H. designed and supervised the research, performed the thermodynamic calculations, developed and analysed the dynamic model, and wrote the paper. M.A., I.H. and E.M.S. performed the experiments and analysed the products. R.P.-B. and Y.F. assisted with analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 550 kb)
Supplementary Information
Supplementary Information (PDF 6893 kb)
Supplementary Information
Supplementary Information (PDF 7708 kb)
Supplementary Information
Supplementary Information (PDF 5442 kb)
Supplementary Information
Supplementary Information (XLSX 46 kb)
Rights and permissions
About this article
Cite this article
Halevy, I., Alesker, M., Schuster, E. et al. A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nature Geosci 10, 135–139 (2017). https://doi.org/10.1038/ngeo2878
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2878
This article is cited by
-
Magnesium silicate chimneys at the Strytan hydrothermal field, Iceland, as analogues for prebiotic chemistry at alkaline submarine hydrothermal vents on the early Earth
Progress in Earth and Planetary Science (2024)
-
Production of Neoproterozoic banded iron formations in a partially ice-covered ocean
Nature Geoscience (2024)
-
Transient fertilization of a post-Sturtian Snowball ocean margin with dissolved phosphate by clay minerals
Nature Communications (2023)
-
Micronutrient availability in Precambrian oceans controlled by greenalite formation
Nature Geoscience (2023)
-
Effect of Ni2+, Zn2+, and Co2+ on green rust transformation to magnetite
Geochemical Transactions (2022)