Abstract
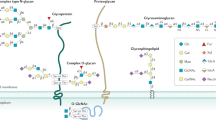
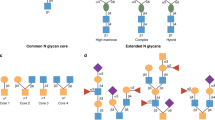
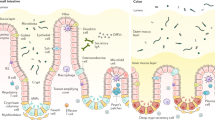
Intestinal epithelial cells apically express glycans, especially α1,2-fucosyl linkages, which work as a biological interface for the host–microbe interaction. Emerging studies have shown that epithelial α1,2-fucosylation is regulated by microbes and by group 3 innate lymphoid cells (ILC3s). Dysregulation of the gene (FUT2) encoding fucosyltransferase 2, an enzyme governing epithelial α1,2-fucosylation, is associated with various human disorders, including infection and chronic inflammatory diseases. This suggests a critical role for an interaction between microbes, epithelial cells and ILC3s mediated via glycan residues. In this Review, using α1,2-fucose and Fut2 gene expression as an example, we describe how epithelial glycosylation is controlled by immune cells and luminal microbes. We also address the pathophysiological contribution of epithelial α1,2-fucosylation to pathogenic and commensal microbes as well as the potential of α1,2-fucose and its regulatory pathway as previously unexploited targets in the development of new therapeutic approaches for human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goto, Y. & Kiyono, H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol. Rev. 245, 147–163 (2012).
Marchiando, A.M., Graham, W.V. & Turner, J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 5, 119–144 (2010).
Johansson, M.E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105, 15064–15069 (2008).
Porter, E.M., van Dam, E., Valore, E.V. & Ganz, T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect. Immun. 65, 2396–2401 (1997).
Salzman, N.H., Ghosh, D., Huttner, K.M., Paterson, Y. & Bevins, C.L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422, 522–526 (2003).
Cash, H.L., Whitham, C.V., Behrendt, C.L. & Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Vaishnava, S. et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Kurashima, Y., Goto, Y. & Kiyono, H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur. J. Immunol. 43, 3108–3115 (2013).
Goto, Y. & Ivanov, I.I. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol. Cell Biol. 91, 204–214 (2013).
Sano, T. et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163, 381–393 (2015).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Honda, K. & Littman, D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 30, 759–795 (2012).
Caballero, S. & Pamer, E.G. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu. Rev. Immunol. 33, 227–256 (2015).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Pham, T.A. et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 16, 504–516 (2014).
Pickard, J.M. et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514, 638–641 (2014).
Aigal, S., Claudinon, J. & Römer, W. Plasma membrane reorganization: A glycolipid gateway for microbes. Biochim. Biophys. Acta 1853, 858–871 (2015).
Imai, M. & Kawaoka, Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr. Opin. Virol. 2, 160–167 (2012).
Marionneau, S. et al. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122, 1967–1977 (2002).
Ohtsubo, K. & Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 (2006).
Rosen, S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Pinho, S.S. & Reis, C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Terahara, K. et al. Distinct fucosylation of M cells and epithelial cells by Fut1 and Fut2, respectively, in response to intestinal environmental stress. Biochem. Biophys. Res. Commun. 404, 822–828 (2011).
Moran, A.P., Gupta, A. & Joshi, L. Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 60, 1412–1425 (2011).
Fukuoka, S., Freedman, S.D. & Scheele, G.A. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc. Natl. Acad. Sci. USA 88, 2898–2902 (1991).
Hase, K. et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 462, 226–230 (2009).
Blondel, C.J. et al. CRISPR/Cas9 screens reveal requirements for host cell sulfation and fucosylation in bacterial type III secretion system-mediated cytotoxicity. Cell Host Microbe 20, 226–237 (2016).
Ma, B., Simala-Grant, J.L. & Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 16, 158R–184R (2006).
Becker, D.J. & Lowe, J.B. Fucose: biosynthesis and biological function in mammals. Glycobiology 13, 41R–53R (2003).
Domino, S.E., Hiraiwa, N. & Lowe, J.B. Molecular cloning, chromosomal assignment and tissue-specific expression of a murine alpha(1,2)fucosyltransferase expressed in thymic and epididymal epithelial cells. Biochem. J. 327, 105–115 (1997).
Domino, S.E., Zhang, L. & Lowe, J.B. Molecular cloning, genomic mapping, and expression of two Secretor blood group a (1,2)fucosyltransferase genes differentially regulated in mouse uterine epithelium and gastrointestinal tract. J. Biol. Chem. 276, 23748–23756 (2001).
Rouquier, S. et al. Molecular cloning of a human genomic region containing the H blood group a(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human Secretor blood group locus. J. Biol. Chem. 270, 4632–4639 (1995).
Avent, N.D. Human erythrocyte antigen expression: its molecular bases. Br. J. Biomed. Sci. 54, 16–37 (1997).
Hoskins, L.C. The ABO blood group antigens and their secretion by healthy and diseased gastric mucosa. Ann. NY Acad. Sci. 140, 848–865 (1967).
Kelly, R.J., Rouquier, S., Giorgi, D., Lennon, G.G. & Lowe, J.B. Sequence and expression of a candidate for the human Secretor blood group a(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 270, 4640–4649 (1995).
Michalski, J.C. & Klein, A. Glycoprotein lysosomal storage disorders: alpha- and beta-mannosidosis, fucosidosis and alpha-N-acetylgalactosaminidase deficiency. Biochim. Biophys. Acta 1455, 69–84 (1999).
Comstock, L.E. & Kasper, D.L. Bacterial glycans: key mediators of diverse host immune responses. Cell 126, 847–850 (2006).
Coyne, M.J., Reinap, B., Lee, M.M. & Comstock, L.E. Human symbionts use a host-like pathway for surface fucosylation. Science 307, 1778–1781 (2005).
Hooper, L.V., Xu, J., Falk, P.G., Midtvedt, T. & Gordon, J.I. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc. Natl. Acad. Sci. USA 96, 9833–9838 (1999).
Kashyap, P.C. et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. USA 110, 17059–17064 (2013).
Tong, M. et al. Reprograming of gut microbiome energy metabolism by the FUT2 Crohn's disease risk polymorphism. ISME J. 8, 2193–2206 (2014).
Pacheco, A.R. et al. Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117 (2012).
Umesaki, Y. & Ohara, M. Factors regulating the expression of the neutral glycolipids in the mouse small intestinal mucosa. Biochim. Biophys. Acta 1001, 163–168 (1989).
Engevik, M.A. et al. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G697–G711 (2013).
Bry, L., Falk, P.G., Midtvedt, T. & Gordon, J.I. A model of host-microbial interactions in an open mammalian ecosystem. Science 273, 1380–1383 (1996).
Nanthakumar, N.N., Dai, D., Newburg, D.S. & Walker, W.A. The role of indigenous microflora in the development of murine intestinal fucosyl- and sialyltransferases. FASEB J. 17, 44–46 (2003).
Hooper, L.V., Bry, L., Falk, P.G. & Gordon, J.I. Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. BioEssays 20, 336–343 (1998).
Nanthakumar, N.N., Meng, D. & Newburg, D.S. Glucocorticoids and microbiota regulate ontogeny of intestinal fucosyltransferase 2 requisite for gut homeostasis. Glycobiology 23, 1131–1141 (2013).
Liu, Z. et al. Mucosal gene expression profiles following the colonization of immunocompetent defined-flora C3H mice with Helicobacter bilis: a prelude to typhlocolitis. Microbes Infect. 11, 374–383 (2009).
Ota, N. et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat. Immunol. 12, 941–948 (2011).
Tumanov, A.V. et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 10, 44–53 (2011).
Kinnebrew, M.A. et al. Interleukin 23 production by intestinal CD103+CD11b+ dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287 (2012).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Goto, Y. et al. IL-10-producing CD4+ T cells negatively regulate fucosylation of epithelial cells in the gut. Sci. Rep. 5, 15918 (2015).
Sawa, S. et al. RORgt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 12, 320–326 (2011).
Hurd, E.A. & Domino, S.E. Increased susceptibility of secretor factor gene Fut2-null mice to experimental vaginal candidiasis. Infect. Immun. 72, 4279–4281 (2004).
Chessa, D., Winter, M.G., Nuccio, S.P., Tükel, C. & Bäumler, A.J. RosE represses Std fimbrial expression in Salmonella enterica serotype Typhimurium. Mol. Microbiol. 68, 573–587 (2008).
Magalhães, A. et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology 19, 1525–1536 (2009).
Magalhães, A. & Reis, C.A. Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors. Braz. J. Med. Biol. Res. 43, 611–618 (2010).
Ruiz-Palacios, G.M., Cervantes, L.E., Ramos, P., Chavez-Munguia, B. & Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1,2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278, 14112–14120 (2003).
Blackwell, C.C. et al. Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet 2, 284–285 (1986).
Blackwell, C.C., Jonsdottir, K., Hanson, M.F. & Weir, D.M. Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet 2, 687 (1986).
Chaim, W., Foxman, B. & Sobel, J.D. Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J. Infect. Dis. 176, 828–830 (1997).
Sheinfeld, J., Schaeffer, A.J., Cordon-Cardo, C., Rogatko, A. & Fair, W.R. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N. Engl. J. Med. 320, 773–777 (1989).
Stapleton, A., Nudelman, E., Clausen, H., Hakomori, S. & Stamm, W.E. Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on histo-blood group secretor status. J. Clin. Invest. 90, 965–972 (1992).
Koda, Y., Soejima, M. & Kimura, H. The polymorphisms of fucosyltransferases. Leg. Med. (Tokyo) 3, 2–14 (2001).
Liu, Y. et al. Extensive polymorphism of the FUT2 gene in an African (Xhosa) population of South Africa. Hum. Genet. 103, 204–210 (1998).
Svensson, L., Petersson, A. & Henry, S.M. Secretor genotyping for A385T, G428A, C571T, C628T, 685delTGG, G849A, and other mutations from a single PCR. Transfusion 40, 856–860 (2000).
Koda, Y., Soejima, M., Liu, Y. & Kimura, H. Molecular basis for secretor type a(1,2)-fucosyltransferase gene deficiency in a Japanese population: a fusion gene generated by unequal crossover responsible for the enzyme deficiency. Am. J. Hum. Genet. 59, 343–350 (1996).
Yu, L.C. et al. Correlation of a missense mutation in the human Secretor α1,2-fucosyltransferase gene with the Lewis(a+b+) phenotype: a potential molecular basis for the weak Secretor allele (Sew). Biochem. J. 312, 329–332 (1995).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
McGovern, D.P. et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum. Mol. Genet. 19, 3468–3476 (2010).
Aghdassi, A.A., Weiss, F.U., Mayerle, J., Lerch, M.M. & Simon, P. Genetic susceptibility factors for alcohol-induced chronic pancreatitis. Pancreatology 15 (Suppl.), S23–S31 (2015).
Folseraas, T. et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 57, 366–375 (2012).
Ishitoya, S., Yamamoto, S., Mitsumori, K., Ogawa, O. & Terai, A. Non-secretor status is associated with female acute uncomplicated pyelonephritis. BJU Int. 89, 851–854 (2002).
Smyth, D.J. et al. FUT2 nonsecretor status links type 1 diabetes susceptibility and resistance to infection. Diabetes 60, 3081–3084 (2011).
Tang, H. et al. A large-scale screen for coding variants predisposing to psoriasis. Nat. Genet. 46, 45–50 (2014).
Xavier, J.M. et al. FUT2: filling the gap between genes and environment in Behçet's disease? Ann. Rheum. Dis. 74, 618–624 (2015).
Xavier, R.J. & Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Miyoshi, J. et al. Ectopic expression of blood type antigens in inflamed mucosa with higher incidence of FUT2 secretor status in colonic Crohn's disease. J. Gastroenterol. 46, 1056–1063 (2011).
Rausch, P. et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl. Acad. Sci. USA 108, 19030–19035 (2011).
Wacklin, P. et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One 6, e20113 (2011).
Lewis, Z.T. et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 3, 13 (2015).
Smilowitz, J.T. et al. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 143, 1709–1718 (2013).
Rayes, A. et al. A genetic modifier of the gut microbiome influences the risk of graft-versus-host-disease and bacteremia following hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. http://dx.doi.org/10.1016/j.bbmt.2015.11.017 (2015).
Azevedo, M. et al. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol. 215, 308–316 (2008).
Borén, T., Falk, P., Roth, K.A., Larson, G. & Normark, S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262, 1892–1895 (1993).
Lindesmith, L. et al. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9, 548–553 (2003).
Imbert-Marcille, B.M. et al. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J. Infect. Dis. 209, 1227–1230 (2014).
Kambhampati, A., Payne, D.C., Costantini, V. & Lopman, B.A. Host genetic susceptibility to enteric viruses: a systematic review and metaanalysis. Clin. Infect. Dis. http://dx.doi.org/10.1093/cid/civ873 (2015).
Payne, D.C. et al. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr. 169, 1040–1045 (2015).
Günaydın, G., Nordgren, J., Sharma, S. & Hammarström, L. Association of elevated rotavirus-specific antibody titers with HBGA secretor status in Swedish individuals: the FUT2 gene as a putative susceptibility determinant for infection. Virus Res. 211, 64–68 (2016).
Umesaki, Y., Okada, Y., Matsumoto, S., Imaoka, A. & Setoyama, H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol. Immunol. 39, 555–562 (1995).
Hooper, L.V. Do symbiotic bacteria subvert host immunity? Nat. Rev. Microbiol. 7, 367–374 (2009).
Katayama, T. et al. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-alpha-L-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol. 186, 4885–4893 (2004).
Rodríguez-Díaz, J., Carbajo, R.J., Pineda-Lucena, A., Monedero, V. & Yebra, M.J. Synthesis of fucosyl-N-acetylglucosamine disaccharides by transfucosylation using a-L-fucosidases from Lactobacillus casei. Appl. Environ. Microbiol. 79, 3847–3850 (2013).
Acknowledgements
This work is financially supported by the Core Research for Evolutional Science and Technology Program of the Japan Science and Technology Agency (JSPS; to H.K.); a Grant-in-Aid for Scientific Research on Innovative Areas (Homeostatic regulation by various types of cell death) (15H01367 to S.U.); a Scientific Research (S) grant (23229004 to H.K.); a Research Activity Start-up grant (15H06159 to Y.G.); a Young Scientists (A) grant (16H06229 to Y.G.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Challenging Exploratory Research grant from the JSPS (to S.U.); a grant (Adjuvant database) from The Ministry of Health, Labor and Welfare (MHLW) and the Japan Agency for Medical Research and Development (AMED) (to S.U. and H.K.); The Uehara Memorial Foundation (to Y.G.); The Naito Foundation (to Y.G.); the Astellas Foundation for Research on Metabolic Disorders (to Y.G.); and the Takeda Science Foundation (to Y.G.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Goto, Y., Uematsu, S. & Kiyono, H. Epithelial glycosylation in gut homeostasis and inflammation. Nat Immunol 17, 1244–1251 (2016). https://doi.org/10.1038/ni.3587
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3587
This article is cited by
-
A non-human primate model for human norovirus infection
Nature Microbiology (2024)
-
Mechanisms of medicinal, pharmaceutical, and immunomodulatory action of probiotics bacteria and their secondary metabolites against disease management: an overview
Folia Microbiologica (2024)
-
FUT2 inhibits the EMT and metastasis of colorectal cancer by increasing LRP1 fucosylation
Cell Communication and Signaling (2023)
-
Intestinal epithelium-specific Fut2 deficiency promotes colorectal cancer through down-regulating fucosylation of MCAM
Journal of Translational Medicine (2023)
-
Intestinal disturbances associated with mortality of children with complicated severe malnutrition
Communications Medicine (2023)