Abstract
Memory T cells promote allograft rejection particularly in co-stimulation blockade–based immunosuppressive regimens. Here we show that the CD2-specific fusion protein alefacept (lymphocyte function–associated antigen-3–Ig; LFA -3–Ig) selectively eliminates memory T cells and, when combined with a co-stimulation blockade–based regimen using cytotoxic T lymphocyte antigen-4 (CTLA-4)-Ig, a CD80- and CD86-specific fusion protein, prevents renal allograft rejection and alloantibody formation in nonhuman primates. These results support the immediate translation of a regimen for the prevention of allograft rejection without the use of calcineurin inhibitors, steroids or pan–T cell depletion.
Similar content being viewed by others
Main
Calcineurin inhibitors and glucocorticosteroids are commonly used to prevent the rejection of allografted organs; however, both agents promote many side effects1. Blockade of the CD28 co-stimulation pathway through inhibition of its ligands, CD80 and CD86 (B7 molecules), has been suggested as a means of preventing allograft rejection without these side effects, and, indeed, the B7–specific fusion protein CTLA4–Ig has been shown to induce permanent engraftment of allografts in some rodent models, particularly when combined with the drug sirolimus, donor-specific transfusion (DST) or both2,3. Unfortunately, CTLA-4–Ig with or without sirolimus is insufficient to prevent rejection in primates4.
Several mechanisms of co-stimulation blockade–resistant rejection have been demonstrated experimentally, many of them implicating T cells with a memory phenotype5. T cells gain this phenotype through previous antigen exposure, heterologous cross-reactivity between alloantigens and environmental pathogens, or homeostatic expansion and thereafter either lack CD28 or otherwise have reduced co-stimulation requirements6,7. We have therefore been interested in developing adjuvant therapies that transiently but specifically neutralize memory T (TM) cells to facilitate the clinical use of co-stimulation blockade in transplantation, preferably adopting maneuvers that avoid TM cell development through homeostatic activation (for example, pan–T cell depletion).
One candidate agent is LFA-3–Ig (also called alefacept), a dimeric fusion protein consisting of the CD2-binding portion of the human lymphocyte function-associated antigen-3 (LFA-3) linked to the Fc portion of human IgG1. LFA-3–Ig is thought to neutralize the effect of CD2-expressing cells through several mechanisms including complement-mediated lysis and interruption of CD2's interactions with LFA-3, limiting helper T cell adhesion to antigen-presenting cells and disrupting the engagement of effector T cell receptor engagement with antigen and major histocompatibility complex molecules. It is currently approved for the clinical treatment of psoriasis, and its therapeutic effect has been linked to its ability to deplete TM cells8,9,10. We therefore investigated LFA-3–Ig as an adjuvant agent for use with the co-stimulation blockade–based regimen of CTLA-4–Ig, sirolimus and/or DST.
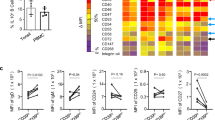
We treated renal allografted rhesus macaques with LFA-3–Ig, CTLA-4–Ig or both weekly for 8 weeks, oral sirolimus daily for 90 d and pretransplant whole blood DST (see Supplementary Methods and Supplementary Fig. 1). Control macaques receiving no treatment, sirolimus alone, sirolimus with DST, sirolimus with DST and CTLA-4–Ig, or sirolimus with DST and LFA-3–Ig had progressively increased survival; however, no macaques remained rejection-free beyond their treatment period (Fig. 1a and Supplementary Table 1), and all macaques developed alloantibody by the onset of rejection (Supplementary Fig. 2). In comparison, when we combined both LFA-3–Ig and CTLA-4–Ig with sirolimus with or without DST, we observed significantly prolonged survival compared to all other treatment groups (Fig. 1a). The groups did not differ by sirolimus level or donor-directed mixed lymphocyte reactivity (Supplementary Table 1). These macaques remained alloantibody-free while receiving both LFA-3–Ig and CTLA-4–Ig (Supplementary Fig. 2), and five of eight macaques receiving the combination therapy remained rejection-free beyond the period of treatment (>90 d) (Fig. 1a). Thus, LFA-3–Ig was clearly additive and potentially synergistic (given that survival seems to exceed that expected for additive effects) with this co-stimulation blockade–based regimen.
(a) Rejection-free survival, defined as the interval between the time of transplantation and the first allograft rejection event shown in days by treatment group. The duration of therapy is shown in the shaded bars along the x-axis. P values were determined by Student's t test (two-tailed) comparing each individual group versus the treatment group receiving LFA-3–Ig, CTLA-4–Ig, sirolimus, and DST. This group had significantly prolonged rejection-free survival compared to all groups except the group receiving the combined therapy without DST. Similarly, the two groups receiving both LFA-3–Ig and CTLA-4–Ig, when considered together, had significantly prolonged survival compared to all other groups combined (P = 0.002, two-tailed Student's t test). (b) PFC analysis of LFA-3–Ig's depletional influence on peripheral blood mononuclear cell T cell subsets: naive (TN; CD4+CD28+CD95low, β7 integrinint and CD8+CD28+CD95low, and CD11alow), central memory (TCM; CD28+CD95+ or CD45RA−CD62L+ in both CD4+ and CD8+ cells) and effector memory (TEM; CD4+CD28−CD95+ or CD45RAhi and CD8+CD28−CD95+ or CD11ahi). Shown is a representative gate defining the three subsets for T cells previously gated for CD3. (c) The influence of LFA-3–Ig treatment on peripheral TEM cell counts, shown for all macaques before transplant, 3 weeks after transplant, and at terminal end points. The mean values for each group are shown as black horizontal bars. (d) Results from PFC experiments performed on freshly isolated peripheral blood lymphocytes obtained from untreated macaques. T cells before treatment have a bimodal expression of CD2 and CD3, with antigen-experienced cells having lower CD3 expression and higher CD2 expression. Shown is this relationship for CD8+ T cells based on PFC plots. The CD3hiCD2lo cells are predominantly TN cells (upper right), whereas the CD3loCD2hi cells are predominantly TEM cells (lower left). TCM cells are less distinctly segregated by these markers. (e) Summary of data from five untreated macaques relating the mean fluorescence intensity (MFI) of CD2 to the surface phenotype for both CD4+ and CD8+ T cells. (f) Renal allograft histological examination of necropsy specimens from a healthy, rejection-free macaque with immunohistochemical (IHC) staining for T cells (blue, CD3+), B cells (brown, CD79+) and macrophages (pink, Ham-56) at low (left) and higher (right) magnification. The Ham-56 macrophage marker is also present on mesangial cells. As a result, glomeruli also stain positive (pink) for Ham-56 (ref. 13). All studies were conducted under protocols approved by the Institutional Animal Care and Use Committees of Emory University or the National Institutes of Health.
We used polychromatic flow cytometry (PFC) to evaluate the effect of LFA-3–Ig treatment on naive and TM cell subsets11 (Fig. 1b, Supplementary Methods and Supplementary Fig. 3). Although there was no gross lymphopenia, there was a general trend toward decreased absolute T cell numbers in LFA-3–Ig–treated macaques, with a greater reduction in CD8+ cells and an increase in the CD4+ to CD8+ ratio that reversed after the withdrawal of LFA-3–Ig compared to macaques not receiving LFA-3–Ig (data not shown). This modest decrease in lymphocyte counts was attributable to a selective depletion in TM cells without alteration of the numbers of naive T cells. In particular, we observed the most substantial depletion within the T effector memory (TEM; CD28−CD95+) subset of CD4+ and CD8+ T cells (Fig. 1c). TEM counts were markedly (P = 0.007, two-tailed Student's t test comparing before and after transplant cell counts) decreased after 3 weeks of LFA-3–Ig therapy. At the time of rejection or killing, TEM counts in LFA-3–Ig–treated macaques had returned to normal (Fig. 1c), suggesting that more prolonged treatment might improve survival. We did not observe this decrease in TEM cells in macaques not treated with LFA-3–Ig (Fig. 1c). Notably, evaluation of CD2 and CD3 expression in peripheral blood T cells from naive macaques indicated that their CD28+ T cells were overwhelmingly CD2loCD3hi, whereas their CD28− T-cells were conversely CD2hi CD3lo, providing an explanation for the TEM selective effects of LFA-3–Ig (Fig. 1d,e). Notably, there was an inverse relationship between the surface expression of CD2 (the target of LFA-3–Ig) and CD28 (the relevant receptor whose ligand binding is blocked by CTLA-4–Ig).
All macaques at one month after transplant showed modest lymphocytic infiltrates in surveillance biopsies studied by multicolor immunohistochemistry (Fig. 1f and Supplementary Methods). Of note, whereas T cell infiltrates were present in all macaques, those macaques that did not receive LFA-3–Ig tended to show a more heterogeneous infiltrate, including monocytes in addition to T cells (Fig. 1f). All macaques reaching the endpoint criteria of depressed renal function (see Supplementary Methods) showed allograft rejection upon histological analysis after necropsy (data not shown). However, we killed two macaques with normally functioning, rejection-free allografts at days 168 and 243 after they developed alloantibody for histological assessment. These macaques' allografts contained focal perivascular aggregates of T and B cells with minimal or no monocytes (Fig. 1f and Supplementary Fig. 4) and without tubulitis or vasculitis. Despite normal renal allograft function and no clinical signs of rejection, we observed multiple focal and highly organized cellular infiltrates. The paucity of monocytes in these infiltrates is consistent with previously reported results of allografts maintaining normal function under co-stimulation blockade–based therapies, in that pure T cell aggregates behaved in a benign fashion, whereas those allografts that contained monocytes were associated with functional impairment12. In comparison, examination of rejecting macaques by an identical IHC staining regimen showed diffuse infiltrates without focal organization and showing greater cellular heterogeneity (data not shown). Notably, there were higher numbers of macrophages observed per objective field in biopsies from rejecting kidneys compared to the infiltrates seen in nonrejecting allografts (data not shown), which has been shown to be of functional importance13.
To investigate the effect of LFA-3–Ig on alloreactive TM cells, we used mixed lymphocyte cultures (MLCs) to specifically evaluate the relationship between the alloresponsiveness of CD4+ and CD8+ T cells and their surface expression of CD2. We labeled responder lymphocytes with carboxyfluorescein succinimidyl ester (CFSE) to enable the identification of responding cells by generation of cell division (Fig. 2a and Supplementary Methods), and we individually examined each division for maturation phenotype and CD2 expression. Responding cells progressively increased CD2 expression, leaving highly alloresponsive cells markedly decorated with the LFA-3–Ig target compared to nonresponding cells (Fig. 2b). Similarly, cells that differentiated into cytokine-producing cells (those producing interferon-γ (IFN-γ)), tumor necrosis factor-α (TNF-α) and/or interleukin-2 (IL-2)) in response to alloantigen stimulation progressively gained CD2 expression commensurate with their acquisition of cytokine secretion capabilities, with the highest CD2 expression in those cells with polyfunctionality (Fig. 2c), cells previously shown to be more robust and terminally differentiated effectors14. In keeping with this target pattern, CTLA-4–Ig and LFA-3–Ig each mediated a dose-dependent inhibition of proliferation for both CD4+ and CD8+ T cells when the fusion proteins were added to MLCs, and the combination induced an additive reduction in the percentage of cells divided across the three logs of concentration (Fig. 2d,e).
(a) CFSE-labeled T cells responding in MLC progressively lose fluorescence intensity by a factor of two, permitting alloresponsive cells to be distinguished from nondividing cells and each generation of division (generation no.) to be separately evaluated by PFC. (b) PFC evaluation of each CFSE generation for accessory molecule expression (CD4 or CD8) and CD2, the target of LFA-3–Ig. (c) Intracellular cytokine staining relating CD2 expression and cytokine secretion (IFN-γ, TNF-α or IL-2) produced after allostimulation. (d,e) Proliferation of CD4+ (d) and CD8+ (e) T cells in CFSE MLC after treatment with CTLA-4–Ig, LFA-3–Ig or both. A dose dependent decrease in proliferation is seen, with the combination therapy having the lowest proliferative capacity at all concentrations tested.
These results show that a combination of LFA-3–Ig, CTLA-4–Ig and sirolimus markedly prolongs renal allograft survival and delays the onset of alloantibody formation in nonhuman primates and that the effect lasts beyond the period of therapy in most macaques. All components of the regimen seem to be involved in its efficacy, as individual agents were ineffective in preventing rejection or antibody formation. The mechanism underlying the salutary effect of LFA-3–Ig seems to be related to increasing CD2 density on alloresponsive cells, particularly CD28− TEM cells, which are least susceptible to co-stimulation blockade and most alloresponsive. These data are consistent with a model in which the transient elimination of co-stimulation blockade–resistant TM cells fosters an environment that more closely resembles that seen in naive murine models. They support the development of a clinically applicable regimen for the prevention of allograft rejection in humans without the use of those therapeutic modalities responsible for the majority of immunosuppressive side effects.
Note: Supplementary information is available on the Nature Medicine website.
References
Meier-Kriesche, H.U. et al. Am. J. Transplant. 6, 1111–1131 (2006).
Lin, H. et al. J. Exp. Med. 178, 1801–1806 (1993).
Li, Y., Zheng, X.X., Li, X.C., Zand, M.S. & Strom, T.B. Transplantation 66, 1387–1388 (1998).
Kirk, A.D. et al. Immunol. Rev. 196, 176–196 (2003).
Valujskikh, A. & Li, X.C. J. Am. Soc. Nephrol. 18, 2252–2261 (2007).
Adams, A.B. et al. J. Clin. Invest. 111, 1887–1895 (2003).
Wu, Z. et al. Nat. Med. 10, 87–92 (2004).
Ortonne, J.P., Lebwohl, M., Em Griffiths, C. & Alefacept Clinical Study Group Eur. J. Dermatol. 13, 117–123 (2003).
Ellis, C.N., Krueger, G.G. & Alefacept Clinical Study Group N. Engl. J. Med. 345, 248–255 (2001).
Chamian, F. et al. Proc. Natl. Acad. Sci. USA 102, 2075–2080 (2005).
Pitcher, C.J. et al. J. Immunol. 168, 29–43 (2002).
Preston, E.H. et al. Am. J. Transplant. 5, 1032–1041 (2005).
Girlanda, R. et al. Am. J. Transplant. 8, 600–607 (2008).
Harari, A. et al. Immunol. Rev. 211, 236–254 (2006).
Acknowledgements
This study was funded in part by the Division of Intramural Research, National Institute of Diabetes, Digestive and Kidney Disease, NIH (Z01 DK062007-06). Salary support for T.A.W. was provided by the Howard Hughes Medical Institute through the NIH Research Scholars Program. A.D.K. is supported by the National Institutes of Health (1U01AI079223-01A1), the Georgia Research Alliance and the McKelvey Foundation. We gratefully acknowledge the expert assistance of J. Bacher and the staff of the NIH Veterinary Pathology Department.
Author information
Authors and Affiliations
Contributions
T.A.W. performed surgical procedures, cared for experimental macaques, conducted in vitro experiments, interpreted data and prepared the manuscript; A.H.C. performed surgical procedures and cared for experimental macaques; A.A. performed surgical procedures, cared for experimental macaques, conducted in vitro experiments and interpreted data; A.P.T. performed surgical procedures, cared for experimental macaques, conducted in vitro experiments and interpreted data; M.R. cared for experimental macaques, conducted in vitro experiments and interpreted data; F.V.L. performed surgical procedures and cared for experimental macaques; R.L.K. conducted in vitro experiments and interpreted data; L.S. conducted in vitro experiments and interpreted data;, M.S. performed histology and immunohistochemistry, interpreted data and prepared the manuscript; C.P.L. interpreted data and prepared the manuscript; A.D.K. conceived of experimental design, performed surgical procedures, cared for experimental macaques, interpreted data and prepared the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figs. 1–4 and Supplementary Methods (PDF 6015 kb)
Rights and permissions
About this article
Cite this article
Weaver, T., Charafeddine, A., Agarwal, A. et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med 15, 746–749 (2009). https://doi.org/10.1038/nm.1993
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1993
This article is cited by
-
Antigen discrimination by T cells relies on size-constrained microvillar contact
Nature Communications (2023)
-
Activation and regulation of alloreactive T cell immunity in solid organ transplantation
Nature Reviews Nephrology (2022)
-
High-Dimensional Renal Profiling: Towards a Better Understanding of Renal Transplant Immune Suppression
Current Transplantation Reports (2019)
-
Allogeneic stem cell transplantation in fully MHC-matched Mauritian cynomolgus macaques recapitulates diverse human clinical outcomes
Nature Communications (2017)
-
Memory T cells in organ transplantation: progress and challenges
Nature Reviews Nephrology (2016)