Abstract
Insulin receptor substrate-1 (IRS-1) and IRS-2 are known to transduce and amplify signals emanating from the insulin receptor1,2,3. Here we show that Grb2-associated binder 1 (Gab1), despite its structural similarity to IRS proteins4, is a negative modulator of hepatic insulin action. Liver-specific Gab1 knockout (LGKO) mice showed enhanced hepatic insulin sensitivity with reduced glycemia and improved glucose tolerance. In LGKO liver, basal and insulin-stimulated tyrosine phosphorylation of IRS-1 and IRS-2 was elevated, accompanied by enhanced Akt/PKB activation. Conversely, Erk activation by insulin was suppressed in LGKO liver, leading to defective IRS-1 Ser612 phosphorylation. Thus, Gab1 acts to attenuate, through promotion of the Erk pathway, insulin-elicited signals flowing through IRS and Akt proteins, which represents a novel balancing mechanism for control of insulin signal strength in the liver.
Similar content being viewed by others
Main
The liver is a major site for glucose uptake, production and storage while hepatic insulin resistance is a crucial factor in the development of hyperglycemia and hypertriglyceridemia in individuals with type 2 diabetes5,6. Therefore, the liver has an important role in both glucose homeostasis and the pathogenesis of diabetes. But it remains to be elucidated how the insulin-initiated signals are modulated in hepatocytes for glucose uptake and metabolism. Insulin receptor substrate-1 (IRS-1) and IRS-2 proteins are known to be required for relay of signals emanating from the insulin receptor, whereas protein tyrosine phosphatase-1B and SH2 domain–containing inositol 5-phosphatase-2 (SHIP-2) act to downregulate insulin signaling1,2,3,7,8. Gab1 (Grb2-associated binder 1) shares structural homology with the IRS family members, possessing a pleckstrin homology domain at the amino terminus, multiple tyrosine phosphorylation sites and proline-rich motifs for interactions with Src homology 2 (SH2)- and SH3-containing proteins4,9. Homozygous Gab1-null mice are embryonically lethal mutation and display multiple defects in growth factor signaling10,11. But it remains to be determined how Gab1 operates in mediating glucose metabolism in adult animals, although previous biochemical analysis in vitro suggested its involvement in insulin action4,12.
To determine the mechanistic role of Gab1 in hepatic insulin action and glucose homeostasis, we set out to generate a tissue-specific knockout mouse with a selective hepatic gene deletion of Gab1. First, using homologous recombination in embryonic stem cells, we created a Gab1flox allele by inserting two loxP sites into introns flanking exon 3 (Fig. 1a,b). Gab1flox/+ mice were bred into albumin-cre (Alb-cre/+) transgenic mice that express the Cre recombinase in hepatocytes from 1 week after birth13. Deletion of exon 3 resulted in removal of amino acid residues 124–198 while also introducing a frameshift mutation that creates a new stop codon at the point of deletion. The LGKO (Gab1flox/flox Alb-cre/+) animals were born at the expected mendelian frequency and were morphologically indistinguishable from the control (Gab1flox/flox) littermates.
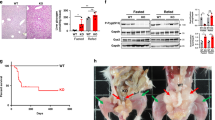
(a) The targeting strategy. loxP sequences are indicated by black triangles. Negative (HSV-TK, PGK-DT) and positive (PGK-NEO) selection markers are indicated by gray bars. The numbered boxes represent exons in the Gab1 gene. The location of the 5´ and 3´ probes used in the Southern analysis are also indicated. TK, thymidine kinase; NEO, neomycin; DT, diphtheria toxin. (b) Southern blot analysis. Three properly targeted embryonic stem cell clones (Gab1flox/+) both contained both wild-type and Gab1flox alleles (F/t). (c) PCR with a and b primers on DNA from Gab1flox/flox (F/F) and LGKO mice. The Gab1flox allele produces a 630 bp fragment and the Gab1− allele produces a 150 bp band. Tissues are as indicated: tail (T), liver (L), skeletal muscle (M), pancreas (P), brown adipose tissue (Ba), white adipose tissue (Wa), hypothalamus (H), pituitary gland (Pi), and kidney (K). (d) Immunoblot analysis of Gab1 protein expression in various tissue isolated from mice of different genotypes: wild-type (WT), Albumin-cre/+ (Cre/+), Gab1flox/flox (F/F), Alb-cre/+ Gab1flox/+ (Cre/+ F/+), and LGKO. Gab1 protein was barely detectable in the liver of LGKO mice. WAT, white adipose tissue. (e) Immunoblot analysis of Gab1 expression in liver and skeletal muscle of control (Gab1flox/flox) and LGKO mice at 2 months and 1 year, respectively. CH, control.
PCR analysis showed an efficient deletion of exon 3 of Gab1 in the liver of LGKO mice, but not in other organs such as skeletal muscle, pancreas, brown and white adipose tissue, hypothalamus, pituitary gland and kidney (Fig. 1c). As shown by immunoblot analysis (Fig. 1d), the Gab1 protein expression was decreased by more than 90% in LGKO liver, but it was unaltered in skeletal muscle, brain and white adipose tissue. The residual Gab1 protein detected in knockout liver lysates may be the result of Gab1 expression in other cell types, such as vascular endothelial cells in the liver, where Alb-Cre is not expressed5,13,14. Stable deficiency of Gab1 was detected in young adult mice (2 months old) and aged (1 year old) mice (Fig. 1e). Deletion of Gab1 did not affect liver development and morphology, and the ratio of liver versus body weight of LGKO mice was normal at 2 months of age (control, 5.21 ± 0.22%, n = 6; LGKO, 5.23 ± 0.13%, n = 9). The body weight for male and female LGKO mice was also similar to controls of the same sex over a 1-year period under fed and fasted conditions (data not shown).
Selective deletion of hepatic Gab1 led to reduced blood glucose levels, ranging from 9.1 to 28.9%, in comparison to both fed and fasted states for gender- and age-matched mice (Fig. 2a). Consistently, serum insulin levels in fed and fasted LGKO male mice were significantly lower than control littermates when measured at 2 months (fed, P = 0.0192; fasted, P = 0.0275) or 1 year (fed, P = 0.0356; fasted, P = 0.0171) (Fig. 2b). We also measured other circulating parameters and liver metabolites (see Supplementary Table 1 online). Plasma free fatty acids were mildly decreased in the mutant mice. Hepatic Gab1 deletion promoted retention of triglycerides in the liver, accompanied by a decrease in levels of circulating triglyceride. Glycogen levels were decreased in 12-month-old LGKO mice, with no differences observed in circulating glyconeogenic precursors.
(a) Blood glucose levels were measured on randomly fed (Fed) or 16-h fasted (Fast) male (M) or female (F) mice; n = 8–39 for each group. (b) Serum insulin levels were measured on fed and fasted male mice; n = 10–13 for each group. (c,d) Glucose tolerance test. Blood glucose (c) and serum insulin (d) during glucose tolerance test performed on 16-h fasted male mice; n = 5–8 for each group. (e) Blood glucose level during insulin tolerance test performed on randomly fed male animals; n = 6–10 per group. *P < 0.05; **P < 0.01; ***P < 0.001 for control versus LGKO. Values are mean ± s.e.m.
After intraperitoneal glucose injection, LGKO mice showed significantly reduced plasma glucose levels for each of the sampling time points during the 120-min glucose tolerance test (Fig. 2c); and thus the area under the glucose curve was significantly decreased (72% of control at 2 months; 59% at 1 year) for LGKO animals as compared to controls. In addition, the insulin response to the glucose load, measured at 60 and 120 min during the glucose tolerance test, was significantly diminished in LGKO animals, with a 44% decrease in the area under the curve (Fig. 2d). Thus, selective deletion of hepatic Gab1 significantly improved glucose tolerance. Coupled with this, the diminished glucose excursion curve, in the face of significantly lower insulin levels, indicates enhanced peripheral insulin sensitivity in the LGKO mice.
To assess the potential difference in peripheral glucose disposal between the genotypes of mice, we first performed in vivo insulin tolerance tests. The ability of insulin to reduce circulating glucose levels was similar between the genotypes at both ages (Fig. 2e). Because the insulin tolerance test is a relatively crude technique for assessment of insulin-stimulated glucose disposal, we subsequently used the more accurate glucose clamp technique to quantitatively assess the glucose disposal rates and hepatic glucose production. Both groups of animals were clamped at the 6-h fasting blood glucose values, 125 ± 2.4 mg/dl. The exogenous glucose infusion rate (GIR) required to maintain euglycemia during the glucose clamp was increased by ∼30% (P = 0.0016) in LGKO mice (Fig. 3a). Similar to results for the insulin tolerance test (Fig. 2e), no significant difference was observed between the genotypes with respect to the ability of insulin to stimulate glucose disposal into skeletal muscle (Fig. 3b). But we did detect more marked suppression of hepatic glucose production from basal during the clamp in LGKO mice (Fig. 3c,d), showing enhanced hepatic insulin sensitivity.
(a) Glucose infusion rate (b) Insulin-stimulated glucose disposal rate (IS-GDR) at the infusion rate of 12 mU/kg/min (c) basal (bas.) and clamped (cl.) hepatic glucose productions (HGP) and (d) insulin suppression of HGP, as determined by euglycemic, hyperinsulinemic clamp analyses. We used 1-year-old male mice; n = 8. *P < 0.05; **P < 0.01 for control versus LGKO. Values are mean ± s.e.m.
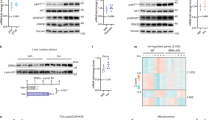
To investigate the molecular basis for enhanced hepatic insulin sensitivity, we examined the activation of Akt/PKB kinase, a critical enzyme in insulin signaling15,16,17,18, by measuring levels of phosphorylated Akt (p-Akt) in both liver and muscle. Tissue lysates were made from the liver and muscle at the indicated time points after insulin administration (Fig. 4a and Supplementary Fig. 1 online). Both basal and insulin-stimulated p-Akt levels were higher in LGKO liver than that in controls, without a change in Akt protein content. Notably, elevated p-Akt levels were detected only in the liver of LGKO mice, with no differences observed in skeletal muscle (Fig. 4a). Tyrosine phosphorylation of the insulin receptor β-subunit (IRβ), normalized to its expression levels, was normal in LGKO liver as well as in muscle (Supplementary Fig. 1 online). Therefore, the increased Akt activity is not caused by enhanced IRβ activation, but probably by a change in downstream signaling. Expression of IRS-1, IRS-2, Shp-2 and the p85α subunit of phosphoinositide 3-kinase (PI3K) in both liver and muscle were normal in LGKO mice (Supplementary Fig. 2 online). However, the general tyrosine phosphorylation levels of both IRS-1 and IRS-2 were detected in LGKO liver, with or without insulin stimulation (Fig. 4b and Supplementary Fig. 3 online). IRS-1 tyrosine phosphorylation was most affected, with a 2.0-fold increase in the basal level and a 1.4-fold increase after insulin stimulation, whereas there was 1.4-fold increase in basal and insulin-stimulated IRS-2 phosphorylation. Increased amounts of IRS-1– and IRS-2–associated p85 were also detected in Gab1-deficient liver. Consistently, we observed enhanced phosphorylation levels of IRS-1 on Tyr608, a major docking site for p85 (Fig. 4b and Supplementary Fig. 3 online). Gab1, unlike the IRS proteins, became weakly (about four-fold) tyrosine phosphorylated after insulin stimulation for 2 min, although high levels of liver Gab1 phosphorylation were induced after administration of epidermal growth factor (EGF) or peroxovanadate injection (Fig. 4c and Supplementary Fig. 3 online). Neither phosphorylation of the p85 binding motif on Gab1 (p-YXXM), nor association of Gab1 with p85 was detected in insulin-treated liver (Fig. 4d), suggesting that Gab1 may not be directly involved in insulin-stimulated PI3K-Akt pathway. Instead, it seems that Gab1 has a negative effect on insulin-stimulated IRS activation and thus, deletion of Gab1 in the liver leads to augmented IRS tyrosine phosphorylation.
(a) p-Akt levels at Ser473 were quantified against Akt protein amounts. Shown are representative immunoblots and statistical data from 3–4 mice, by setting 100 for the control value at 2 min. (b) Upper left graph: relative tyrosine phosphorylation (PY) levels of IRS-1 and -2, in the liver (n = 3). Lower left graph: relative amounts of p85α binding to IRS-1 and -2, in the liver after insulin treatment (n = 2–3). Right graph: relative levels of p-IRS-1 Tyr608 in the liver for insulin treatment (n = 3). (c) Tyrosine phosphorylation of Gab1 in the liver was detected 2 min after insulin injection into the vena cava. (d) Gab1 phosphorylation on the p-YXXM motifs and its association with p85α in response to saline solution, insulin (Ins), EGF or peroxovanadate. (e) Insulin-induced p-Erk1/2 and p-IRS-1 Ser612 were shown together with Erk2 and IRS-1 blots as loading controls. In (c) and (e), two representative samples of each condition are displayed.
Injection of insulin into the vena cava led to activation of extracellular signal-regulated kinase (Erk) in the liver (Fig. 4e). But the insulin-stimulated Erk activity was abolished in Gab1-deficient hepatocytes (Fig. 4e), suggesting that Gab1 has a crucial role in mediating insulin-triggered Erk activation. It was previously reported that serine phosphorylation of IRS-1 by Erk can downregulate IRS-1 tyrosine phosphorylation and association with p85, although the physiological significance was not known19,20,21. Using an antibody specific for phosphorylated Ser612 (p-IRS-1 Ser612), we detected insulin-induced phosphorylation of IRS-1 on Ser612, a known negative regulatory site on IRS-1 for insulin signaling20,21. Notably, this serine phosphorylation event was abolished in Gab1-deficient liver, correlating well with the suppression of Erk activation (Fig. 4e). Taken together, these results suggest that although Gab1 does not directly mediate insulin-induced PI3K-Akt activation, this adapter protein is essential for insulin-stimulated Erk activation with subsequent serine phosphorylation of IRS-1, which leads to attenuation of IRS-1 tyrosine phosphorylation and downregulation of the PI3K-Akt pathway in hepatocytes. We propose that this role of Gab1 constitutes a negative feedback loop regulating insulin signal strength in the liver. The precise role of PI3K in modulating insulin signaling and glucose metabolism seems more complicated than previously thought22,23,24, and this new mouse model provides a valuable tool for further examination of this complex mechanism. Notably, promotion of the Erk pathway by Gab1 in hepatocytes has a negative effect on insulin-regulated glucose metabolism, but a positive role in proliferative signaling as revealed by its requirement for liver regeneration (E.A.B-C., S.L., N. Droin, E.E.Z., T.V. Nguyen & G.-S.F., unpublished data). Thus, pharmaceutical interference of Gab1 activity may be considered in designing new strategies for hepatic insulin sensitization.
Methods
Creation of a conditional Gab1 mutant allele.
Genomic DNA fragments of Gab1 were isolated from a λDASHII mouse genomic library, and a targeting construct was engineered using a triple-loxP system (provided by R. Premont, Duke University). We flanked exon 3 with by two loxP sites (which codes for amino acids 124–198). We introduced the linearized construct DNA into R1 embryonic stem cells by electroporation and screened G418-resistant cell colonies for homologous recombination by PCR and reconfirmed our results with Southern blot analysis. Embryonic stem cell clones with a loxP-floxed exon 3 (Gab1flox) without the TK-neo cassette were obtained by transient transfection with a CMV-Cre construct (pBS185). The engineered embryonic stem cells were injected into C57BL/6 blastocysts and chimeric mice were obtained. Germline transmission of the Gab1flox allele was obtained from three independent embryonic stem cell clones.
Generation of LGKO mice.
The Gab1flox allele was generated in 129/Sv background and mutant mice were crossed with wild-type C57BL/6 animals for four generations to acquire the C57BL/6 background. Gab1flox/flox mice were crossed with Alb-cre transgenic mice (C57BL/6-TgN (Alb-cre)21Mgn)14, to generate LGKO mice (Gab1flox/flox Alb-cre/+). The Gab1flox/flox littermates were used as controls. We housed all animals in a virus-free facility on a 12-h light-dark cycle and fed them a standard mouse food. The Burnham Institute Animal Research Committee approved all protocols for animal use. Genotyping was done by PCR analysis on genomic DNA extracted from mouse tails, using the following primers: Cre primers 5´-GCCTGCATTACCGGTCGATGCAACGA-3´, 5´-GTGGCAGATGGCGCGGCAACACCATT-3´ and Gab1flox/t primers: 5´-GGTGAATCGACGGGTGC-TTGTGA-3´, 5´-CAGATTGGCCTTGAACTGGTAAG-3´. We also performed PCR to detect the specific deletion of exon 3 in the liver by analyzing genomic DNAs extracted from various tissue and organs, using a pair of primers (a: 5´-ACAAGTGTGAGTGTGCGCACATG-3´; b: 5´-CAGATTGGCCTTGAACTGGTAAG-3´) that produce a 630 base pair fragment for the Gab1flox allele and a 150 base pair fragment for the Gab1− allele.
Physiological assays.
The following measures were performed on randomly fed or on 16-h fasted animals. We measured blood glucose levels on whole venous blood using an automatic glucose monitor (One Touch Basic, Lifescan). Serum insulin levels were measured by ELISA, using rat insulin as a standard (Crystal chem). Serum free fatty acids were quantified using the NEFA-C Kit (Wako Chemicals). We assessed serum β-hydroxybutyrates using the β-hydroxy-butyrate kit from Catachem. Serum triglyceride levels were analyzed using a kit from Linco. Circulating lactate was quantified in serum using lactate reagent and standards from Sigma. Hepatic glycogen contents were analyzed as previously described25. Liver triglycerides were extracted as previously described26, and triglyceride content was determined by a GPO Kit from Pointe Scientific Inc. We performed a glucose tolerance test on 16-h fasted animals; followed by injection intraperitoneally with D-glucose (2 g/kg body weight, Sigma). Insulin tolerance test was performed on randomly fed animals injected intraperitoneally with human insulin (1 U/kg body weight, Lilly). Statistical analysis of the data was performed using a two-tailed unpaired t-test.
Euglycemic clamp studies were performed on mice at 1 year of age as previously described27. Plasma glucose–specific activity was measured after deproteinization with barium hydroxide and zinc sulfate as previously described28. We calculated hepatic glucose production and glucose disposal rate at basal state and during the 30-min steady-state portion of the glucose clamp. Tracer determined rates were quantified using the Steele equation for steady-state conditions29. Comparisons between the two groups were conducted using analysis variance (ANOVA),
Biochemical analysis.
For insulin stimulation, 2-month-old male mice were fasted for 16 h, and anesthetized with intraperitoneal injection of Avertin (0.015 ml/g). Human insulin (5 U, Lilly), recombinant murine epidermal growth factor (EGF) (100 μg, Calbiochem) or peroxovanadate (300 μl) was injected into the inferior vena cava. We harvested liver and skeletal muscle at indicated time points and quickly froze them in liquid nitrogen. Frozen tissue samples were homogenized in protein lysis buffer30 and clarified by centrifugation at 37,000g for 1 h at 4 °C. We determined protein concentration using a Bio-Rad kit. Immunoprecipitation and immunoblotting analysis were performed following standard procedures and the signals were detected by an enhanced chemiluminescence kit (Amersham). Rabbit polyclonal antibody against Gab1 was produced using a purified glutathione S-transferase fusion protein of Gab1 following standard procedures. Antibodies to phosphorylated Akt (Ser473), Akt, phosphorylated Erk1/2, phosphorylated-(Tyr) p85 of PI3K, phosphorylated IRS-1 (Ser612) were obtained from Cell Signaling. Antibodies to p85α, p110α, IRβ and Erk2 were from Santa Cruz and antibody specific for phosphotyrosine was purchased from Upstate Biotechnology Inc. Antibody to p-IRS-1 (Tyr608) was from Calbiochem, and antibodies to IRS-1 and IRS-2 were provided by M. White (Harvard University). Immunoblots were scanned and signals were quantified using ImageQuant software. Statistical analysis of the data was performed using a two-tailed unpaired t-test.
Note: Supplementary information is available on the Nature Medicine website.
References
Tamemoto, H. et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372, 182–186 (1994).
Araki, E. et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372, 186–190 (1994).
Withers, D.J. et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904 (1998).
Holgado-Madruga, M., Emlet, D.R., Moscatello, D.K., Godwin, A.K. & Wong, A.J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379, 560–564 (1996).
Michael, M.D. et al. Loss of insulin signalling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6, 87–97 (2000).
Saltiel, A.R. & Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Elchebly, M. et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999).
Clement, S. et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409, 92–97 (2001).
Gu, H. & Neel, B.G. The “Gab” in signal transduction. Trends Cell Biol. 13, 122–130 (2003).
Itoh, M. et al. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 20, 3695–3704 (2000).
Sachs, M. et al. Essential role of Gab1 for signalling by the c-Met receptor in vivo. J. Cell Biol. 150, 1375–1384 (2000).
Rocchi, S. et al. Determination of Gab1 (Grb2-associated binder-1) interaction with insulin receptor-signalling molecules. Mol. Endocrinol. 12, 914–923 (1998).
Postic, C. & Magnuson, M.A. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 (2000).
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999).
Jiang, Z.Y. et al. Insulin signalling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 100, 7569–7574 (2003).
Krook, A., Roth, R.A., Jiang, X.J., Zierath, J.R. & Wallberg-Henriksson, H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes 47, 1281–1286 (1998).
Summers, S.A. & Birnbaum, M.J. A role for the serine/threonine kinase, Akt, in insulin-stimulated glucose uptake. Biochem. Soc. Trans. 25, 981–988 (1997).
Cho, H. et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 (2001).
Mothe, I. & Van Obberghen, E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J. Biol. Chem. 271, 11222–11227 (1996).
De Fea, K. & Roth, R.A. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36, 12939–12947 (1997).
De Fea, K. & Roth, R.A. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J. Biol. Chem. 272, 31400–31406 (1997).
Fruman, D.A. et al. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat. Genet. 26, 379–382 (2000).
Ueki, K. et al. Positive and negative roles of p85alpha and p85beta regulatory subunits of phosphoinositide 3-kinase in insulin signalling. J. Biol. Chem. 278, 48453–48466 (2003).
Terauchi, Y. et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat. Genet. 21, 230–235 (1999).
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. & Smith, F.J. Colorimetri method for the determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Frayn, K.N. & Maycock, P.F. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J. Lipid Res. 21, 139–144 (1980).
Miles, P.D., Barak, Y., He, W., Evans, R.M. & Olefsky, J.M. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J. Clin. Invest. 105, 287–392 (2000).
Revers, R.R., Fink, R., Griffin, J., Olefsky, J.M. & Kolterman, O.G. Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J. Clin. Invest. 73, 664–672 (1984).
Steele, R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann. NY Acad. Sci. 82, 420–430 (1959).
Sun, Y. et al. Role of Gab1 in UV-induced c-Jun NH2-terminal kinase activation and cell apoptosis. Mol. Cell. Biol. 24, 1531–1539 (2004).
Acknowledgements
We thank R. Premont for triple-loxP constructs, M. White for antibodies to IRS-1 and IRS-2, R. Abraham for providing the lactate kit, and colleagues for discussion. This work was supported by US National Institutes of Health grants DK60484 to A.L.H., DK33651 to J.M.O. and GM53660 to G.S.F. G.S.F. was a recipient of a career development award from the American Diabetes Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Insulin-induced tyrosine phosphorylation of IRβ in LGKO liver. (PDF 57 kb)
Supplementary Fig. 2
Normal expression levels of proteins involved in insulin signalling. (PDF 48 kb)
Supplementary Fig. 3
Deletion of Gab1 leads to enhanced insulin signalling through IRS-1, -2 in hepatocytes. (PDF 49 kb)
Supplementary Table 1
Characteristics of mutant mice at 2 and 12 months of age (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Bard-Chapeau, E., Hevener, A., Long, S. et al. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat Med 11, 567–571 (2005). https://doi.org/10.1038/nm1227
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1227
This article is cited by
-
Gab2 deficiency suppresses high-fat diet-induced obesity by reducing adipose tissue inflammation and increasing brown adipose function in mice
Cell Death & Disease (2021)
-
Insulin receptor endocytosis in the pathophysiology of insulin resistance
Experimental & Molecular Medicine (2020)
-
The epigenetic landscape of transgenerational acclimation to ocean warming
Nature Climate Change (2018)
-
Scaffolding protein Gab1 regulates myeloid dendritic cell migration in allergic asthma
Cell Research (2016)
-
Cardiac Gab1 deletion leads to dilated cardiomyopathy associated with mitochondrial damage and cardiomyocyte apoptosis
Cell Death & Differentiation (2016)