Abstract
Prion diseases are caused by an unconventional infectious agent termed prion, composed mainly of the misfolded prion protein (PrPSc)1. The development of highly sensitive assays for biochemical detection of PrPSc in blood is a top priority for minimizing the spread of the disease2. Here we show that the protein misfolding cyclic amplification (PMCA) technology3 can be automated and optimized for high-efficiency amplification of PrPSc. We show that 140 PMCA cycles leads to a 6,600-fold increase in sensitivity over standard detection methods. Two successive rounds of PMCA cycles resulted in a 10 million–fold increase in sensitivity and a capability to detect as little as 8,000 equivalent molecules of PrPSc. Notably, serial PMCA enables detection of PrPSc in blood samples of scrapie-afflicted hamsters with 89% sensitivity and 100% specificity. These findings represent the first time that PrPSc has been detected biochemically in blood, offering promise for developing a noninvasive method for early diagnosis of prion diseases.
Similar content being viewed by others
Main
Although prion diseases are relatively rare in humans, the recent appearance of a new clinicopathologic phenotype, termed variant Creutzfeldt-Jakob disease, and the experimental evidence that this phenotype is causally linked to the causative agent of bovine spongiform encephalopathy4,5,6 have raised concern about a possible outbreak of a large epidemic in the human population. Over the past few years, bovine spongiform encephalopathy has become a significant health problem affecting many countries7, and it is now apparent that variant Creutzfeldt-Jakob disease can be iatrogenically transmitted from human to human by blood transfusion8,9.
The central event in the pathogenesis of prion diseases is the formation of a protease-resistant misfolded protein named PrPSc, which is a conformationally modified version of a normal protein, termed PrPC (ref. 10). PrPSc is the only validated surrogate marker for the disease, but its concentration is high enough for routine biochemical detection only in the brain and some lymphoid tissues2. One important aim in diagnosis of prion disease is the biochemical detection of PrPSc in blood, a fluid known to contain infective agents8,11,12. Although much effort has been devoted to detect prions in blood, thus far all attempts to develop a reproducible biochemical detection assay using blood samples have failed because the quantity of PrPSc in this fluid is very small.
With the aim of facilitating biochemical detection of PrPSc, we have developed a new technique that enables PrPSc amplification in the test tube3. This method, termed protein misfolding cyclic amplification (PMCA), is based on conversion of large amounts of PrPC triggered by undetectable quantities of PrPSc (refs. 3,13). In a cyclic manner and conceptually analogous to PCR cycling, PrPSc is incubated with excess PrPC to enlarge the PrPSc aggregates, which are then sonicated to generate multiple smaller units for the continued formation of new PrPSc (ref. 3). We and others have previously reported that PMCA enables an increase of sensitivity for PrPSc detection between 10- and 60-fold3,14,15,16,17 and the technology was applied to replicate the misfolded protein from diverse species18. The newly generated protein shows the same biochemical and structural properties as brain-derived PrPSc and, notably, it is infectious to wild-type animals, producing a disease with characteristics similar to those of the illness produced by brain-isolated prions19.
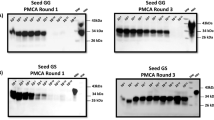
The application of the PMCA technology for large-scale biochemical diagnosis in blood depends upon designing an automated, high-throughput system that will enable an increase of sensitivity of three to five orders of magnitude over existing technologies. With this aim, we have developed an automated PMCA system in which the sonication is carried out in a microplate horn block sonicator that can be programmed for automatic operation. This modification decreases cross-contamination, as tubes remain sealed during the process and there is no probe immersion in the samples. Nevertheless, the key feature of the new machine is that it enables tests to be performed automatically and to include routinely large numbers of cycles. As reported before, the efficiency of amplification increases exponentially with the number of cycles3. We analyzed the sensitivity of detection after automated PMCA by comparing the signal intensity in western blots before and after amplification. Performing 140 cycles of PMCA enabled detection of PrPSc in as little as a 6.6 million–fold dilution of scrapie brain infected with the 263K prion strain (Fig. 1a). An equivalent signal of PrPSc was detected without PMCA in a 1,000-fold dilution of the same material, indicating that the increase of sensitivity under these conditions was approximately 6,600-fold (Fig. 1a). We did not detect any PrPSc signal when we subjected normal brain homogenate to the same 140 PMCA cycles, but without PrPSc inoculum (Fig. 1a). During these experiments, we noted that the efficiency of amplification started to decrease after ∼150 cycles (75 h of incubation). This problem is likely to be the result of a negative effect of maintaining the material under continuous incubation at 37 °C on the stability of PrPC substrate or other brain cofactors essential to catalyze the conversion. We based this conclusion on an experiment in which the amplification efficiency was substantially reduced when we preincubated the 10% normal brain homogenate (with or without sonication) during 75 h before the beginning of PMCA amplifications (data not shown). Therefore, to further increase sensitivity of detection, we carried out two successive rounds of PMCA cycling by diluting the amplified samples into fresh 10% normal brain homogenate (Fig. 1b). The experiment consisted of performing a first round of 96 PMCA cycles in which PrPSc signal was detected up to the 3.1 × 106-fold dilution of scrapie-afflicted brain. Thereafter, we diluted this and all the successive dilutions in which we had not detected any PrPSc signal 10-fold into normal brain homogenate and subjected the samples to a new round of 118 PMCA cycles. This second round of PMCA enabled detection of PrPSc up to the 5 × 1010-fold dilution of scrapie-afflicted hamster brain (Fig. 1b), which correspond to ∼0.02 mean lethal dose (LD50). By comparing the signal intensity of PrPSc with and without PMCA, the increase of detection sensitivity over standard western blot was around 10 million–fold. Our estimations of the quantity of PrPSc detected by serial automated PMCA (saPMCA) indicate that two rounds of PMCA detected as little as 20 fg/ml, or as few as 4 × 105 equivalent molecules of PrPSc per milliliter. Because we used a volume of 20 μl for our studies, we estimated that we were detecting approximately 8,000 equivalent molecules of PrPSc. We performed these calculations by estimating the quantity of PrPSc in the brain homogenate by using western blot and ELISA and comparing the signal with known concentrations of recombinant PrP. The saPMCA procedure can be repeated several times to reach even greater sensitivity in samples containing tiny amounts of PrPSc. Control experiments in which samples of normal brain homogenates were subjected to the same series of PMCA cycles, but in the absence of scrapie brain inoculum, did not show any signal of protease-resistant PrPSc (Fig. 1b). Moreover, even after as many as 20 rounds of saPMCA, we did not detect any PrPSc signal in the absence of initial inoculum (data not shown).
(a) Aliquots of 263K scrapie brain were subjected to the serial dilutions indicated in the figure, which were prepared into 10% normal brain homogenate. Samples were either immediately frozen (nonamplified samples; upper panel) or subjected to 140 PMCA cycles (lower panel). PrPSc reactivity was detected after digestion with proteinase K using western blot. (b) As in a, scrapie brain was diluted serially into 10% normal brains and either kept frozen (upper panel) or amplified by 96 PMCA cycles (middle panel). Starting with amplified samples corresponding to the 3.1 × 106 dilution of scrapie brain, all the remaining amplified samples were further diluted 10-fold into fresh normal brain homogenate and subjected to another round of 118 PMCA cycles (lower panel). Again PrPSc signal was detected by western blotting after digestion with proteinase K. NBH, normal brain homogenate. –PrPSc corresponds to the control experiment in which scrapie brain homogenate was not added to the samples. All samples were treated with proteinase K (PK) before electrophoresis, except those in which –PK is indicated.
Thus far, the only method that could detect prions in blood of some animals is the infectivity bioassay11,20,21. But the use of bioassays for widespread diagnosis is limited by the length of time that it takes to obtain results (several months to years) and the species barrier effect. Nevertheless, these experiments enabled us to estimate that the buffy coat fraction from 1 ml of blood obtained from scrapie-afflicted hamsters contains approximately 1–10 LD50, which is equivalent to 0.1–1 pg or between 2 × 106 and 2 × 107 molecules of PrPSc (refs. 2,11).
To evaluate the application of saPMCA for detection of prions in blood, we obtained samples from 12 normal healthy control hamsters and 18 hamsters with clinical signs of scrapie disease induced by intracerebral inoculation of infected brain. We extracted the buffy coat from 1 ml of fresh blood, centrifuged it and added it to 10% normal hamster brain homogenate. After 144 PMCA cycles, 1 of the 18 scrapie samples showed a signal corresponding to amplified PrPSc (Fig. 2). After a second round of 144 cycles of PMCA, we observed PrPSc in nine of the scrapie samples, but none of the control samples. After a total of six rounds of PMCA, 16 of the 18 scrapie samples gave a clear positive signal, whereas none of the 12 control samples showed any detectable signal (Fig. 2). These results indicate that saPMCA enables detection of prions in blood with 89% sensitivity and 100% specificity (i.e., no false positives). We did not detect any signal in control samples of blood even after 13 rounds of saPMCA (data not shown), indicating that the procedure is very specific for detection of prions in infected samples. Thus far, the only assay capable of detecting prions in blood is the animal bioassay and its sensitivity for PrPSc detection in hamster blood is ∼31%, which corresponds to an average of various experiments reported by different investigators11. Therefore, saPMCA has a substantially higher sensitivity than even the most sensitive bioassay. Furthermore, because no other method can detect prions in blood, it is yet not clear whether all sick animals are expected to contain prions in their blood. Interestingly, we observed no correlation between the quantities of PrPSc detected in the brain of these animals, with the difficulty of detecting PrPSc in blood measured as the number of rounds of serial PMCA needed to amplify the protein to detectable levels (Fig. 3). These data suggest that the quantity of PrPSc in blood varies among different animals. Whether PrPSc in blood comes from brain leakage is presently unknown and currently under investigation. But if this is the case, our data suggest that the rate of leakage varies among different animals.
Blood samples from 18 clinically sick hamsters and 12 control hamsters were taken. We used 1 ml of blood to prepare buffy coat. The section corresponding to buffy coat was subjected to freezing-thawing three times and centrifuged at 100,000g for 1 h at 4 °C. The pellet was resuspended directly on 100 μl of 10% normal brain homogenate. Samples were subjected to 144 cycles of PMCA. We used 20 μl of the sample for detection of PrPSc by western blot after digestion with proteinase K. We diluted 8 μl into 72 μl of normal brain homogenate and performed a new round of 144 PMCA cycles. We repeated this process several times. Each panel represents the results obtained in each round of PMCA. S, samples from sick animals; C, samples from control animals. We treated all samples with proteinase K (PK) before electrophoresis, except the normal brain homogenate (NBH) in which –PK is indicated.
We estimated the concentration of PrPSc in brain by western blot after proteinase K digestion in each of the 18 hamsters used in Figure 2 to detect PrPSc in blood. We based the estimation on the comparison of the signal in western blots with known concentrations of recombinant PrP. In the graph, PrPSc concentration in brain is plotted against the number of rounds of PMCA needed to first detect PrPSc signal in the blood of the same hamsters. ND, not detected.
Our findings represent the first time that prions have been biochemically detected in blood. The high level of sensitivity and specificity indicate that saPMCA offers promise for the design of a sensitive biochemical test for blood diagnosis of transmissible spongiform encephalopathies. One of the key factors that enables the considerable increase of amplification rate as compared to our previously reported PMCA technology is the automation of the system, which allows the consistent running of a much larger number of cycles. Also important is the discovery that addition of fresh substrate tissue between serial rounds of PMCA leads to a substantial boost in amplification. Finally, some small modifications in the solution conditions (addition of EDTA and removal of SDS from the conversion buffer) and the preparation of the brain homogenates have been important to obtain high levels of amplification. In its current state, saPMCA needs several days to detect PrPSc with high sensitivity, which may raise questions regarding its practical applicability for large-scale blood diagnosis. There are several easy-to-implement improvements that may substantially decrease the time needed to obtain results and may further increase sensitivity. PMCA is not a detection system, but rather an amplification step that can be coupled with any high-efficiency technology to detect PrPSc. For these studies we used western blot, which is the most standard and reliable assay to visualize PrPSc, but also one of the least sensitive. In addition, because the misfolded protein detected after amplification is the one we added as part of the substrate tissue, the protein can be labeled using any radioactive, fluorescent or tagging procedure to enable high-sensitivity detection. Finally, the automated PMCA procedure is amenable to robotization for high-throughput screening of samples.
We are currently studying the detection of prions in blood from infected animals during the presymptomatic phase as well as detection of PrPSc in plasma and other blood fractions. The implementation of a similar blood-detection procedure for human and cattle samples will undoubtedly contribute to minimizing the risk of infection with agents causing transmissible spongiform encephalopathies and will have a tremendous impact on the beef industry, safety of blood banks and plasma products, estimation of the variant Creutzfeldt-Jakob disease epidemic and diagnosis of disease. An early and sensitive diagnosis is also important from the treatment perspective, as it would enable therapeutic intervention to start at an early stage of the disease, before the appearance of clinical signs and permanent brain damage.
Methods
Preparation of tissue homogenates.
We perfused control and scrapie-afflicted hamsters with PBS plus 5 mM EDTA before harvesting tissue. We prepared 10% brain homogenates (wt/vol) in conversion buffer (PBS containing 150 mM NaCl, 1.0% Triton X-100, 4 mM EDTA and the Complete Protease Inhibitor Cocktail from Roche). We clarified the samples using a brief, low-speed centrifugation (1,500 r.p.m. for 30 s) in an Eppendorf centrifuge. We made dilutions of this brain homogenate in conversion buffer and dilutions are expressed in relation to the brain (for example, a 100-fold dilution is equivalent to a 1% brain homogenate). We discovered that the brain homogenate used as substrate should not be kept for >24 h at 4 °C and freezing-thawing should be avoided. We also highly recommend that the brain homogenate be stored at −80 °C instead of −20 °C. Although the inoculum does not require special storage procedures, we recommend that it be stored frozen at −80 °C. It is possible to freeze-thaw it at least 20 times without significant loss of efficiency.
In vivo infectivity studies.
We used Syrian Golden hamsters as an experimental model of scrapie. We inoculated hamsters when they were 4–6 weeks old. We stereotaxically injected anesthetized hamsters in the right hippocampus with 1 μl of the sample as previously described22. Hamsters at advanced stages of the disease were killed using exposure to carbon dioxide to avoid excessive pain. We extracted brains and homogenized them as described above. We titrated the scrapie infectious material and obtained 1 LD50 from a brain dilution of approximately 1 × 109.
Blood preparation.
We collected blood from normal and scrapie-afflicted hamsters directly from the heart using a syringe containing EDTA. We placed blood in tubes containing sodium citrate and separated the blood into aliquots of 1 ml. Thereafter, we added 1 ml of PBS and separated the buffy coat by a Ficoll gradient using standard procedures. We extracted the buffy coat fraction, subjected it to three consecutive cycles of freezing and thawing to break cells and centrifuged it at 100,000g for 1 h at 4 °C. We resuspended the pellet directly in 100 μl of normal brain homogenate and subjected it to PMCA cycling.
PMCA procedure.
Although the principle of PMCA remains the same as previously described3, we have optimized and automated the system, thus enabling the routine processing of many samples through a large number of PMCA cycles to reach higher amplification efficiency. We mixed aliquots of normal and scrapie brain homogenate prepared in conversion buffer in a final volume of 80 μl and loaded them into 0.2 ml PCR tubes. For amplification of blood samples, we directly resuspended the buffy coat prepared as previously described in 10% normal brain homogenate. We immediately froze controls at −80 °C, and positioned tubes containing the samples to be amplified on an adaptor placed on the plate holder of a microsonicator (Misonix Model 3000) and programmed it to perform incubation cycles 30 min in length at 37 °C followed by a 40 s pulse of sonication set at 60–80% potency19. We kept the microplate horn in an incubator set at 37 °C throughout the whole process and thus the incubation was performed without shaking. A more detailed technical protocol for automated PMCA, including a troubleshooting section, has been recently described23,24.
Protease resistance assay.
We incubated samples with 50 μg/ml of proteinase K for 60 min at 45 °C with shaking. We stopped the digestion by adding electrophoresis sample buffer.
Western blot.
We fractionated proteins by SDS-PAGE under reducing conditions, electroblotted them into nitrocellulose membrane, and probed them with 3F4 antibody (Signet) diluted 1:5,000 in PBS, 0.05% Tween-20. We visualized the immunoreactive bands by enhanced chemiluminescence assay (Amersham). We analyzed western blot signals by densitometry, using a UVP Bioimaging system EC3 apparatus.
PrPSc quantification.
To estimate the quantity of PrPSc present in the 10% scrapie brain homogenates, we analyzed several dilutions of scrapie brain homogenate by western blot in the same gel as aliquots of known amounts of recombinant hamster PrP. We evaluated the signal intensity by densitometry and estimated the quantity of PrP in the sample by extrapolation of the calibration curve prepared with recombinant PrP. To minimize artifacts due to saturated or weak signal, we measured several different dilutions and analyzed each dilution in triplicate. To standardize the signal among the different blots, the densitometric data were expressed relative to the value of the signal of the same quantity of normal brain homogenate (without proteinase K treatment). We confirmed PrPSc quantification by ELISA using recombinant PrP as the standard. We estimated the number of molecules of PrPSc detected by mathematical calculation of the dilution and the known concentration of PrPSc in the brain homogenate, and this estimation is expressed as equivalent molecules to emphasize the fact that the number is obtained by comparison with detection of recombinant PrP.
Change history
11 January 2013
In the version of this article initially published, there was a mistake in Figure 2. Several bands depicted in the second round of protein misfolding cyclic amplification were inadvertently duplicated in the third round. The error has been corrected in the HTML and PDF versions of the article.
References
Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 95, 13363–13383 (1998).
Soto, C. Diagnosing prion diseases: needs, challenges and hopes. Nat. Rev. Microbiol. 2, 809–819 (2004).
Saborio, G.P., Permanne, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Cousens, S.N., Vynnycky, E., Zeidler, M., Will, R.G. & Smith, R.G. Predicting the CJD epidemic in humans. Nature 385, 197–198 (1997).
Collinge, J. Variant Creutzfeldt-Jakob disease. Lancet 354, 317–323 (1999).
Bruce, M.E. et al. Transmissions to mice indicate that new variant CJD is caused by the BSE agent. Nature 389, 498–501 (1997).
Bradley, R. & Liberski, P.P. Bovine spongiform encephalopathy (BSE): the end of the beginning or the beginning of the end? Folia Neuropathol. 42, Suppl. A, 55–68 (2004).
Llewelyn, C.A. et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363, 417–421 (2004).
Peden, A.H., Head, M.W., Ritchie, D.L., Bell, J.E. & Ironside, J.W. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364, 527–529 (2004).
Cohen, F.E. & Prusiner, S.B. Pathologic conformations of prion proteins. Ann. Rev. Biochem. 67, 793–819 (1998).
Brown, P., Cervenakova, L. & Diringer, H. Blood infectivity and the prospects for a diagnostic screening test in Creutzfeldt-Jakob disease. J. Lab. Clin. Med. 137, 5–13 (2001).
Houston, F., Foster, J.D., Chong, A., Hunter, N. & Bostock, C.J. Transmission of BSE by blood transfusion in sheep. Lancet 356, 999–1000 (2000).
Soto, C., Saborio, G.P. & Anderes, L. Cyclic amplification of protein misfolding: application to prion-related disorders and beyond. Trends Neurosci. 25, 390–394 (2002).
Bieschke, J. et al. Autocatalytic self-propagation of misfolded prion protein. Proc. Natl. Acad. Sci. USA 101, 12207–12211 (2004).
Deleault, N.R., Lucassen, R.W. & Supattapone, S. RNA molecules stimulate prion protein conversion. Nature 425, 717–720 (2003).
Piening, N., Weber, P., Giese, A. & Kretzschmar, H. Breakage of PrP aggregates is essential for efficient autocatalytic propagation of misfolded prion protein. Biochem. Biophys. Res. Commun. 326, 339–343 (2005).
Barret, A. et al. Evaluation of quinacrine treatment for prion diseases. J. Virol. 77, 8462–8469 (2003).
Soto, C. et al. Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 579, 638–642 (2005).
Castilla, J., Saá, P., Hetz, C. & Soto, C. In vitro generation of infectious scrapie prions. Cell 121, 195–206 (2005).
Brown, P. et al. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 38, 810–816 (1998).
Ingrosso, L., Vetrugno, V., Cardone, F. & Pocchiari, M. Molecular diagnostics of transmissible spongiform encephalopathies. Trends Mol. Med. 8, 273–280 (2002).
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J. & Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 22, 5435–5445 (2003).
Castilla, J., Saá, P. & Soto, C. Cyclic Amplification of Prion Protein Misfolding. in Techniques in Prion Research (Methods and Tools in Biosciences and Medicine) (eds. Lehmann, S. & Grassi, J.) 198–213 (Birkhauser, Basel, Switzerland, 2004).
Saa, P., Castilla, J. & Soto, C. Cyclic Amplification of Protein Misfolding and Aggregation. in Amyloid Proteins: Methods and Protocols (ed. Sigurdsson, E.M.) 53–65 (Humana Press, Totowa, New Jersey, 2004).
Acknowledgements
We would like to thank K. Maundrell (Serono Pharmaceutical Research Institute, Geneva, Switzerland) for bringing to our attention the automatic sonicator. C.S. is part of the European Community project TSELAB. This research was supported in part by US National Institutes of Health grants AG0224642 and NS049173 and the Intramural John Sealy Endowed Fund for Biomedical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have applied for a patent, which discloses the findings reported in the study.
Rights and permissions
About this article
Cite this article
Castilla, J., Saá, P. & Soto, C. Detection of prions in blood. Nat Med 11, 982–985 (2005). https://doi.org/10.1038/nm1286
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1286
This article is cited by
-
Dynamics of CWD prion detection in feces and blood from naturally infected white-tailed deer
Scientific Reports (2023)
-
Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid
Nature Protocols (2023)
-
PMCA for ultrasensitive detection of prions and to study disease biology
Cell and Tissue Research (2023)
-
Generation of human chronic wasting disease in transgenic mice
Acta Neuropathologica Communications (2021)
-
Effect of the micro-environment on α-synuclein conversion and implication in seeded conversion assays
Translational Neurodegeneration (2020)