Abstract
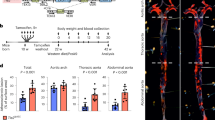
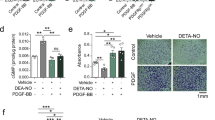
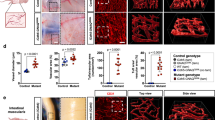
R-Ras is a small GTPase of the Ras family that regulates cell survival and integrin activity. Despite a number of in vitro studies, the in vivo function of R-Ras remains unclear. Here, we used R-Ras–null mice to explore the in vivo function of this small GTPase. Our results show a role for R-Ras as a regulator of vascular differentiation that primarily affects the remodeling of blood vessels. We show that R-Ras–null mice, although otherwise phenotypically normal, mount excessive vascular responses. We found that in vivo R-Ras expression is largely confined to fully differentiated smooth muscle cells, including those of blood vessels, and to endothelial cells. Challenging the R-Ras–null mice with arterial injury or tumor implantation showed exaggerated neointimal thickening in response to the injury and increased angiogenesis in the tumors. In wild-type mice, R-Ras expression was greatly reduced in hyperplastic neointimal smooth muscle cells and in angiogenic endothelial cells. Forced expression of activated R-Ras suppressed mitogenic and invasive activities of growth factor–stimulated vascular cells. These results establish an unexpected role for R-Ras in blood vessel homeostasis and suggest that R-Ras signaling may offer a target for therapeutic intervention in vascular diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sethi, T., Ginsberg, M.H., Downward, J. & Hughes, P.E. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol. Biol. Cell 10, 1799–1809 (1999).
Cole, A.L., Subbanagounder, G., Mukhopadhyay, S., Berliner, J.A. & Vora, D.K. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler. Thromb. Vasc. Biol. 23, 1384–1390 (2003).
Ehrhardt, A., Ehrhardt, G.R., Guo, X. & Schrader, J.W. Ras and relatives–job sharing and networking keep an old family together. Exp. Hematol. 30, 1089–1106 (2002).
Wang, B., Zou, J.X., Ek-Rylander, B. & Ruoslahti, E. R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J. Biol. Chem. 275, 5222–5227 (2000).
Zou, J.X. et al. An Eph receptor regulates integrin activity through R-Ras. Proc. Natl Acad. Sci. USA 96, 13813–13818 (1999).
Zou, J.X., Liu, Y., Pasquale, E.B. & Ruoslahti, E. Activated SRC oncogene phosphorylates R-ras and suppresses integrin activity. J. Biol. Chem. 277, 1824–1827 (2002).
Zhang, Z., Vuori, K., Wang, H., Reed, J.C. & Ruoslahti, E. Integrin activation by R-ras. Cell 85, 61–69 (1996).
Hughes, P.E. et al. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 88, 521–530 (1997).
Suzuki, J., Kaziro, Y. & Koide, H. Positive regulation of skeletal myogenesis by R-Ras. Oncogene 19, 1138–1146 (2000).
Ramocki, M.B. et al. Signaling through mitogen-activated protein kinase and Rac/Rho does not duplicate the effects of activated Ras on skeletal myogenesis. Mol. Cell. Biol. 17, 3547–3555 (1997).
Saez, R., Chan, A.M., Miki, T. & Aaronson, S.A. Oncogenic activation of human R-ras by point mutations analogous to those of prototype H-ras oncogenes. Oncogene 9, 2977–2982 (1994).
Oinuma, I., Ishikawa, Y., Katoh, H. & Negishi, M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305, 862–865 (2004).
Arai, A., Nosaka, Y., Kohsaka, H., Miyasaka, N. & Miura, O. CrkL activates integrin-mediated hematopoietic cell adhesion through the guanine nucleotide exchange factor C3G. Blood 93, 3713–3722 (1999).
Ratajska, A., Zarska, M., Quensel, C. & Kramer, J. Differentiation of the smooth muscle cell phenotypes during embryonic development of coronary vessels in the rat. Histochem. Cell Biol. 116, 79–87 (2001).
Carmeliet, P., Moons, L. & Collen, D. Mouse models of angiogenesis, arterial stenosis, atherosclerosis and hemostasis. Cardiovasc. Res. 39, 8–33 (1998).
Clowes, A.W., Reidy, M.A. & Clowes, M.M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab. Invest. 49, 327–333 (1983).
Sata, M. et al. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J. Mol. Cell. Cardiol. 32, 2097–2104 (2000).
Duckers, H.J. et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 7, 693–698 (2001).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Jain, R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Moiseeva, E.P. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc. Res. 52, 372–386 (2001).
Weissberg, P.L., Grainger, D.J., Shanahan, C.M. & Metcalfe, J.C. Approaches to the development of selective inhibitors of vascular smooth muscle cell proliferation. Cardiovasc. Res. 27, 1191–1198 (1993).
Jin, G. et al. Effects of active and negative mutants of Ras on rat arterial neointima formation. J. Surg. Res. 94, 124–132 (2000).
Meadows, K.N., Bryant, P., Vincent, P.A. & Pumiglia, K.M. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene 23, 192–200 (2004).
Koyama, N. et al. Regulation and function of an activation-dependent epitope of the beta 1 integrins in vascular cells after balloon injury in baboon arteries and in vitro. Am. J. Pathol. 148, 749–761 (1996).
Zambrowicz, B.P. et al. Disruption and sequence identification of 2,000 genes in mouse embryonic stem cells. Nature 392, 608–611 (1998).
Wang, H.G. et al. R-Ras promotes apoptosis caused by growth factor deprivation via a Bcl-2 suppressible mechanism. J. Cell Biol. 129, 1103–1114 (1995).
Ishida, T. et al. Targeted disruption of endothelial cell-selective adhesion molecule inhibits angiogenic processes in vitro and in vivo. J. Biol. Chem. 278, 34598–35604 (2003).
Reynolds, L.E. et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8, 27–34 (2002).
Naldini, L., Blomer, U., Gage, F.H., Trono, D. & Verma, I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl Acad. Sci. USA 93, 11382–11388 (1996).
Acknowledgements
We thank E. Engvall, D. Hanahan and Y. Yamaguchi for discussions and comments on the manuscript, S. Krajewski for help with immunohistology, M. Sata for providing a tutorial video on arterial wire injury, and R. Varghese for editing. This work was supported by US National Cancer Institute grants PO1 CA82713, RO1 CA79984 and RO1 CA098162 (to E.R.); T32 CA09579 (to M.K.); and P30 CA 30199 (Cancer Center Support Grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Disruption of Rras mRNA and the absence of protein expression in R-Ras–null mice. (PDF 244 kb)
Supplementary Fig. 2
Immunohistological detection of R-Ras expression in various mouse tissues. (PDF 409 kb)
Supplementary Fig. 3
Spatiotemporal pattern of R-Ras expression during mouse development. (PDF 321 kb)
Supplementary Fig. 4
Immunohistological characterization of neointimal lesions. (PDF 248 kb)
Supplementary Fig. 5
R-Ras expression is downregulated significantly in cultured vascular cells. (PDF 208 kb)
Rights and permissions
About this article
Cite this article
Komatsu, M., Ruoslahti, E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat Med 11, 1346–1350 (2005). https://doi.org/10.1038/nm1324
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1324
This article is cited by
-
Prostaglandin E2 breaks down pericyte–endothelial cell interaction via EP1 and EP4-dependent downregulation of pericyte N-cadherin, connexin-43, and R-Ras
Scientific Reports (2020)
-
Transcriptome analysis identified a novel 3-LncRNA regulatory network of transthyretin attenuating glucose induced hRECs dysfunction in diabetic retinopathy
BMC Medical Genomics (2019)
-
The inhibition of Bax activation-induced apoptosis by RasGRP2 via R-Ras-PI3K-Akt signaling pathway in the endothelial cells
Scientific Reports (2019)
-
R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature
Nature Communications (2017)
-
Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis
Scientific Reports (2016)