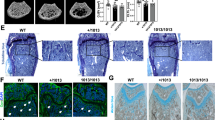
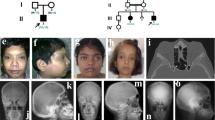
Abstract
The spontaneous mouse grey-lethal (gl) mutation is responsible for a coat color defect and for the development of the most severe autosomal recessive form of osteopetrosis. Using a positional cloning approach, we have mapped and isolated the gl locus from a ∼1.5 cM genetic interval. The gl locus was identified in a bacterial artificial chromosome (BAC) contig by functional genetic complementation in transgenic mice. Genomic sequence analysis revealed that the gl mutation is a deletion resulting in complete loss of function. The unique ∼3 kb wild-type transcript is expressed primarily in osteoclasts and melanocytes as well as in brain, kidney, thymus and spleen. The gl gene is predicted to encode a 338–amino acid type I transmembrane protein that localizes to the intracellular compartment. Mutation in the human GL gene leads to severe recessive osteopetrosis. Our studies show that mouse Gl protein function is absolutely required for osteoclast and melanocyte maturation and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Karsenty, G. The genetic transformation of bone biology. Genes Dev. 13, 3037–3051 (1999).
Teitelbaum, S.L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).
Lazner, F., Gowen, M., Pavasovic, D. & Kola, I. Osteopetrosis and osteoporosis: two sides of the same coin. Hum. Mol. Genet. 8, 1839–1846 (1999).
Reeves, J.D., August, C.S., Humbert, J.R. & Weston, W.L. Host defense in infantile osteopetrosis. Pediatrics 64, 202–206 (1979).
Wilson, C.J. & Vellodi, A. Autosomal recessive osteopetrosis: diagnosis, management, and outcome. Arch. Dis. Child 83, 449–452 (2000).
Gerritsen, E.J. et al. Bone marrow transplantation for autosomal recessive osteopetrosis. A report from the Working Party on Inborn Errors of the European Bone Marrow Transplantation Group. J. Pediatr. 125, 896–902 (1994).
Eapen, M., Davies, S.M., Ramsay, N.K. & Orchard, P.J. Hematopoietic stem cell transplantation for infantile osteopetrosis. Bone Marrow Transplant. 22, 941–946 (1998).
Frattini, A. et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 25, 343–346 (2000).
Sobacchi, C. et al. The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum. Mol. Genet. 10, 1767–1773 (2001).
Kornak, U. et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215 (2001).
Tondravi, M.M. et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81–84 (1997).
Iotsova, V. et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 3, 1285–1289 (1997).
Yoshida, H. et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 (1990).
Dai, X.M. et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120 (2002).
Johnson, R.S., Spiegelman, B.M. & Papaioannou, V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71, 577–586 (1992).
Wang, Z.Q. et al. Bone and haematopoietic defects in mice lacking c-fos. Nature 360, 741–745 (1992).
Kong, Y.Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Begg, S.K. et al. Delayed hematopoietic development in osteopetrotic (op/op) mice. J. Exp. Med. 177, 237–242 (1993).
Thesingh, C.W. & Scherft, J.P. Fusion disability of embryonic osteoclast precursor cells and macrophages in the microphthalmic osteopetrotic mouse. Bone 6, 43–52 (1985).
Hodgkinson, C.A. et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74, 395–404 (1993).
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Lomaga, M.A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Li, Y.P., Chen, W., Liang, Y., Li, E. & Stashenko, P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 23, 447–451 (1999).
Scimeca, J.C. et al. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26, 207–213 (2000).
Gruneberg, H. Grey-lethal, a new mutation in the house mouse. J. Heredity 27, 105–109 (1936).
Walker, D.G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 190, 784–785 (1975).
Rajapurohitam, V. et al. The mouse osteopetrotic grey-lethal mutation induces a defect in osteoclast maturation/function. Bone 28, 513–523 (2001).
Vacher, J. & Bernard, H. Genetic localization and transmission of the mouse osteopetrotic grey-lethal mutation. Mamm. Genome 10, 239–243 (1999).
Chalhoub, N., Benachenhou, N. & Vacher, J. Physical and transcriptional map of the mouse Chromosome 10 proximal region syntenic to human 6q16-q21. Mamm. Genome 12, 887–892 (2001).
Zhang, Q.H. et al. Cloning and functional analysis of cDNAs with open reading frames for 300 previously undefined genes expressed in CD34+ hematopoietic stem/progenitor cells. Genome Res. 10, 1546–1560 (2000).
Brown, D., Hirsch, S. & Gluck, S. Localization of a proton-pumping ATPase in rat kidney. J. Clin. Invest. 82, 2114–2126 (1988).
Perou, C.M. et al. Identification of the murine beige gene by YAC complementation and positional cloning. Nat. Genet. 13, 303–308 (1996).
Baron, R., Neff, L., Louvard, D. & Courtoy, P.J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 101, 2210–2222 (1985).
Baron, R. et al. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J. Cell Biol. 106, 1863–1872 (1988).
Blair, H.C., Teitelbaum, S.L., Ghiselli, R. & Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245, 855–857 (1989).
Palokangas, H., Mulari, M. & Vaananen, H.K. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J. Cell Sci. 110, 1767–1780 (1997).
Vacher, J. & Tilghman, S.M. Dominant negative regulation of the mouse α-fetoprotein gene in adult liver. Science 250, 1732–1735 (1990).
Bennett, D.C., Cooper, P.J. & Hart, I.R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer 39, 414–418 (1987).
Emerson, J.A., Vacher, J., Cirillo, L.A., Tilghman, S.M. & Tyner, A.L. The zonal expression of α-fetoprotein transgenes in the livers of adult mice. Dev. Dyn. 195, 55–66 (1992).
Marcinkiewicz, M., Savaria, D. & Marcinkiewicz, J. The pro-protein convertase PC1 is induced in the transected sciatic nerve and is present in cultured Schwann cells: comparison with PC5, furin and PC7, implication in pro-BDNF processing. Mol. Brain Res. 59, 229–246 (1998).
Acknowledgements
The authors thank R. McInnes and J. Horsford for melan-a cells; S. Breton for V-ATPase antibodies; D. Bennett for discussion; D. Lohnes and R. Baron for critical reading of the manuscript; U. Ramenghi and families for their participation; J. Marcinkiewicz for advice; and H. Bernard for technical assistance. M.F. has a studentship from the Fonds pour la Formation de Chercheurs et d'Aide à la Recherche (FCAR). This work was supported by a grant from the Canadian Institutes of Health Research and by Hoechst Marion Roussel grant no. R97047 to J.V., partially supported by grant MIUR-FIRB #RBNE019J9W, and is manuscript #71 of the Genoma2000/ITB Project funded by Fondazione Cariplo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chalhoub, N., Benachenhou, N., Rajapurohitam, V. et al. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med 9, 399–406 (2003). https://doi.org/10.1038/nm842
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm842