Abstract
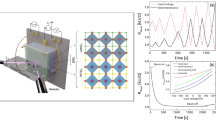
Room-temperature ionic liquids (RTILs) are new materials with fundamental importance for energy storage and active lubrication. They are unusual liquids, which challenge the classical frameworks of electrolytes, whose behaviour at electrified interfaces remains elusive, with exotic responses relevant to their electrochemical activity. Using tuning-fork-based atomic force microscope nanorheological measurements, we explore here the properties of confined RTILs, unveiling a dramatic change of the RTIL towards a solid-like phase below a threshold thickness, pointing to capillary freezing in confinement. This threshold is related to the metallic nature of the confining materials, with more metallic surfaces facilitating freezing. This behaviour is interpreted in terms of the shift of the freezing transition, taking into account the influence of the electronic screening on RTIL wetting of the confining surfaces. Our findings provide fresh views on the properties of confined RTIL with implications for their properties inside nanoporous metallic structures, and suggests applications to tune nanoscale lubrication with phase-changing RTILs, by varying the nature and patterning of the substrate, and application of active polarization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Armand, M., Endres, F., MacFarlane, D. R., Ohno, H. & Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 8, 621–629 (2009).
Uesugi, E., Goto, H., Eguchi, R., Fujiwara, A. & Kubozono, Y. Electric double-layer capacitance between an ionic liquid and few-layer graphene. Sci. Rep. 3, 1595 (2013).
Palacio, M. & Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 40, 247–268 (2010).
Dold, C., Amann, T. & Kailer, A. Influence of electric potentials on friction of sliding contacts lubricated by an ionic liquid. Phys. Chem. Chem. Phys. 17, 10339–10342 (2015).
Smith, A. M., Lovelock, K. R. J., Gosvami, N. N., Welton, T. & Perkin, S. Quantized friction across ionic liquid thin films. Phys. Chem. Chem. Phys. 15, 15317–15320 (2013).
Siria, A. et al. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 494, 455–458 (2013).
Bocquet, L. & Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 39, 1073–1095 (2010).
Secchi, E., Niguès, A., Jubin, L., Siria, A. & Bocquet, L. Scaling behavior for ionic transport and its fluctuations in individual carbon nanotubes. Phys. Rev. Lett. 116, 154501 (2016).
Secchi, E. et al. Massive radius-dependent flow slippage in single carbon nanotubes. Nature 537, 210–213 (2016).
Agrawal, K. V., Shimizu, S., Drahushuk, L. W., Kilcoyne, D. & Strano, M. S. Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes. Nat. Nanotech. 12, 267–273 (2017).
Fedorov, M. V. & Kornyshev, A. A. Ionic liquids at electrified interfaces. Chem. Rev. 114, 2978–3036 (2014).
Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 11, 306–310 (2012).
Perkin, S., Salanne, M., Madden, P. & Lynden-Bell, R. Is a Stern and diffuse layer model appropriate to ionic liquids at surfaces? Proc. Natl Acad. Sci. USA 110, E4121 (2013).
Atkin, R. et al. AFM and STM studies on the surface interaction of [BMP]TFSA and (EMIm]TFSA ionic liquids with Au(111). J. Phys. Chem. C 113, 13266–13272 (2009).
Rotenberg, B. & Salanne, M. Structural transitions at ionic liquids interfaces. J. Phys. Chem. Lett. 6, 4978–4985 (2015).
Endres, F., Borisenko, N., El Abedin, S. Z., Hayes, R. & Atkin, R. The interface ionic liquid(s)/electrode(s): in situ STM and AFM measurements. Faraday Discuss. 154, 221–233 (2012).
Borisenko, N., Atkin, R., Lahiri, A., El Abedin, S. Z. & Endres, F. Effect of dissolved LiCl on the ionic liquid-Au(111) interface: an in situ STM study. J. Phys. Condens. 26, 284111 (2014).
Liu, Y., Zhang, Y., Wu, G. & Hu, J. Coexistence of liquid and solid phases of Bmim-PF6 ionic liquid on mica surfaces at room temperature. J. Am. Chem. Soc. 128, 7456–7457 (2006).
Bovio, S., Podestà, A., Milani, P., Ballone, P. & Del Pópolo, M. G. Nanometric ionic-liquid films on silica: a joint experimental and computational study. J. Phys. Condens. Matter 21, 424118 (2009).
Bovio, S., Podesta, A., Lenardi, C. & Milani, P. Evidence of extended solidlike layering in [Bmim][NTf2] ionic liquid thin films at room-temperature. J. Phys. Chem. B 113, 6600–6603 (2009).
Yokota, Y., Harada, T. & Fukui, K. I. Direct observation of layered structures at ionic liquid/solid interfaces by using frequency-modulation atomic force microscopy. Chem. Commun. 46, 8627–8629 (2010).
Lee, A. A. & Perkin, S. Ion-image interactions and phase-transition at electrolyte-metal interface. J. Phys. Chem. Lett. 7, 2753–2757 (2016).
Ueno, K., Kasuya, M., Watanabe, M., Mizukami, M. & Kurihara, K. Resonance shear measurement of nanoconfined ionic liquids. Phys. Chem. Chem. Phys. 12, 4066–4071 (2010).
Jurado, L. A. et al. Irreversible structural change of a dry ionic liquid under nanoconfinement. Phys. Chem. Chem. Phys. 17, 13613–13624 (2015).
Bou-Malham, I. & Bureau, L. Nanoconfined ionic liquids: effect of surface charges on flow and molecular layering. Soft Matter 6, 4062–4065 (2010).
Niguès, A., Siria, A., Vincent, P., Poncharal, P. & Bocquet, L. Ultra-high interlayer friction inside boron-nitride nanotubes. Nat. Mater. 13, 688–693 (2014).
O’Mahony, A. M., Silvester, D. S., Aldous, L., Hardacre, C. & Compton, R. G. Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J. Chem. Eng. Data 53, 2884–2891 (2008).
Morita, M., Ohmi, T., Hasegawa, E., Kawakami, M. & Ohwada, M. Growth of native oxide on a silicon surface. J. Appl. Phys. 68, 1272–1281 (1990).
Anson, F. C. & Lingane, J. J. Chemical evidence for oxide films on platinum electrometric electrodes. J. Am. Chem. Soc. 79, 4901–4904 (1957).
Smith, A. M., Lee, A. A. & Perkin, S. The electrostatic screening length in concentrated electrolytes increases with concentration. J. Phys. Chem. Lett. 7, 2157–2163 (2016).
Gebbie, M. A. et al. Long range electrostatic forces in ionic liquids. Chem. Commun. (2017).
Leroy, S. & Charlaix, E. Hydrodynamic interactions for the measurement of thin film elastic properties. J. Fluid Mech. 674, 389–407 (2011).
Elbourne, A. et al. Nanostructure of the ionic liquid—graphite stern layer. ACS Nano 9, 7608–7620 (2015).
Buchner, F., Forster-Tonigold, K., Bozorgchenani, M., Gross, A. & Jürgen Behm, R. Interaction of a self assembled ionic liquid layer with graphite (0001): a combined experimental and theoretical study. J. Phys. Chem. Lett. 7, 226–233 (2016).
Alba-Simionesco, C. et al. Effects of confinement on freezing and melting. J. Phys. Condens. Matter 18, R15 (2006).
Bhatt, V. D., Gohi, K. & Mishra, A. Thermal energy storage capacity of some phase changing materials and ionic liquids. Int. J. ChemTech Res. 2, 1771e9 (2010).
Mahan, G. D. Many-Particle Physics (Springer Science and Business Media, 2000).
Dibrov, S. M. & Koch, J. K. Crystallographic view of fluidicstructures for room-temperatureionic liquids: 1-butyl-3-methyl-imidazolium hexafluorophosphat. ActaCryst. C 62, o19-o21 (2006).
Alam, M. T., Islam, M. M., Okajima, T. & Ohsaka, T. Capacitance measurements in a series of room-temperature ionic liquids at glassy carbon and gold electrode interfaces. J. Phys. Chem. C 112, 16600–16608 (2008).
Sweeney, J. et al. Control of nanoscale friction on gold in an ionic liquid by a potential-dependent ionic lubricant layer. Phys. Rev. Lett. 109, 155502 (2012).
Li, H., Wood, R. J., Rutland, M. W. & Atkin, R. An ionic liquid lubricant enables superlubricity to be switched in situ using an electrical potential. Chem. Commun. 50, 4368–4370 (2014).
Fajardo, O. Y., Bresme, F., Kornyshev, A. A. & Urbakh, M. Electrotunable lubricity with ionic liquid nanoscale films. Sci. Rep. 5, 7698 (2015).
Fajardo, O. Y., Bresme, F., Kornyshev, A. A. & Urbakh, M. Electrotunable friction with ionic liquid lubricants: how important is the molecular structure of the ions? J. Phys. Chem. Lett. 6, 3998–4004 (2015).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nat. Mater. 13, 387–393 (2014).
Kondrat, S., Georgi, N., Fedorov, M. V. & Kornyshev, A. A. A superionic state in nano-porous double-layer capacitors: insights from Monte Carlo simulations. Phys. Chem. Chem. Phys. 13, 11359–11366 (2011).
Kondrat, S. & Kornyshev, A. Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys. Condens. Matter 23, 022201 (2010).
Acknowledgements
L.B. and A.S. thank B. Rotenberg, B. Cross and E. Charlaix for many fruitful discussions. J.C., A.N. and A.S. acknowledge funding from the European Union’s H2020 Framework Programme/ERC Starting Grant agreement number 637748 - NanoSOFT. L.B. acknowledges support from the European Union’s FP7 Framework Programme/ERC Advanced Grant Micromegas. L.B. acknowledges funding from a PSL chair of excellence. The authors acknowledge funding from ANR project BlueEnergy.
Author information
Authors and Affiliations
Contributions
A.S. and L.B. conceived and directed the research. J.C. performed the experiments and analysed the data. A.N., L.B. and A.S., supervised the experiments. V.K., B.C., A.S. and L.B. conducted the theoretical analysis and B.C. performed the molecular simulations. All authors contributed to the scientific discussions and the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1256 kb)
Rights and permissions
About this article
Cite this article
Comtet, J., Niguès, A., Kaiser, V. et al. Nanoscale capillary freezing of ionic liquids confined between metallic interfaces and the role of electronic screening. Nature Mater 16, 634–639 (2017). https://doi.org/10.1038/nmat4880
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4880
This article is cited by
-
A review of current understanding in tribochemical reactions involving lubricant additives
Friction (2023)
-
Electronic screening using a virtual Thomas–Fermi fluid for predicting wetting and phase transitions of ionic liquids at metal surfaces
Nature Materials (2022)
-
Electrotunable friction with ionic liquid lubricants
Nature Materials (2022)
-
Ionic liquids on uncharged and charged surfaces: In situ microstructures and nanofriction
Friction (2022)
-
Probing the nanofriction of non-halogenated phosphonium-based ionic liquid additives in glycol ether oil on titanium surface
Friction (2022)