Abstract
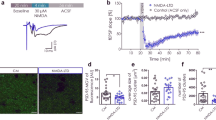
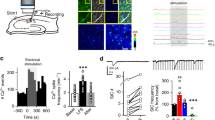
In the CA1 region of the rat hippocampus, long-term potentiation (LTP) requires the activation of NMDA receptors (NMDARs) and leads to an enhancement of AMPA receptor (AMPAR) function. In neonatal hippocampus, this increase in synaptic strength seems to be mediated by delivery of AMPARs to the synapse. Here we studied changes in surface expression of native AMPA and NMDA receptors following induction of LTP in the adult rat brain. In contrast to early postnatal rats, we find that LTP in the adult rat does not alter membrane association of AMPARs. Instead, LTP leads to rapid surface expression of NMDARs in a PKC- and Src-family-dependent manner. The present study suggests a developmental shift in the LTP-dependent trafficking of AMPA receptors. Moreover, our results indicate that insertion of NMDA receptors may be a key step in regulating synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shi, S. H. et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284, 1811–1816 (1999).
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000).
Petralia, R. S. et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat. Neurosci. 2, 31–36 (1999).
Nusser, Z. et al. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21, 545–559 (1998).
Racca, C., Stephenson, F. A., Streit, P., Roberts, J. D. & Somogyi, P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J. Neurosci. 20, 2512–2522 (2000).
Lan, J. et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 4, 382–390 (2001).
Lu, W. Y. et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat. Neurosci. 2, 331–338 (1999).
Huang, Y. et al. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29, 485–496 (2001).
O'Dell, T. J., Kandel, E. R. & Grant, S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature 353, 558–560 (1991).
Hall, R. A. & Soderling, T. R. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience 78, 361–371 (1997).
Hall, R. A. & Soderling, T. R. Differential surface expression and phosphorylation of the N-methyl-D-aspartate receptor subunits NR1 and NR2 in cultured hippocampal neurons. J. Biol. Chem. 272, 4135–4140 (1997).
Nayak, A. S., Moore, C. I. & Browning, M. D. Ca2+/calmodulin-dependent protein kinase II phosphorylation of the presynaptic protein synapsin I is persistently increased during long-term potentiation. Proc. Natl. Acad. Sci. USA 93, 15451–15456 (1996).
Nayak, A., Zastrow, D. J., Lickteig, R., Zahniser, N. R. & Browning, M. D. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature 394, 680–683 (1998).
Pickard, L., Noel, J., Henley, J. M., Collingridge, G. L. & Molnar, E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J. Neurosci. 20, 7922–7931 (2000).
Mammen, A. L., Huganir, R. L. & O' Brien, R. J. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J. Neurosci. 17, 7351–7358 (1997).
Dunah, A. W. & Standaert, D. G. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J. Neurosci. 21, 5546–5558 (2001).
David, V., Hochstenbach, F., Rajagopalan, S. & Brenner, M. B. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin). J. Biol. Chem. 268, 9585–9592 (1993).
Miyatani, S. et al. Neural cadherin: role in selective cell–cell adhesion. Science 245, 631–635 (1989).
Bashir, Z. I., Alford, S., Davies, S. N., Randall, A. D. & Collingridge, G. L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature 349, 156–158 (1991).
Aniksztejn, L. & Ben-Ari, Y. Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J. Neurophysiol. 74, 2349–2357 (1995).
Kauer, J. A., Malenka, R. C. & Nicoll, R. A. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron 1, 911–917 (1988).
Lau, L. F. & Huganir, R. L. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J. Biol. Chem. 270, 20036–20041 (1995).
Grosshans, D. R. & Browning, M. D. Protein kinase C activation induces tyrosine phosphorylation of the NR2A and NR2B subunits of the NMDA receptor. J. Neurochem. 76, 737–744 (2001).
Rosenblum, K., Dudai, Y. & Richter-Levin, G. Long-term potentiation increases tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit 2B in rat dentate gyrus in vivo. Proc. Natl. Acad. Sci. USA 93, 10457–10460 (1996).
Rosenblum, K., Berman, D. E., Hazvi, S., Lamprecht, R. & Dudai, Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J. Neurosci. 17, 5129–5135 (1997).
Parfitt, K. D. & Madison, D. V. Phorbol esters enhance synaptic transmission by a presynaptic, calcium-dependent mechanism in rat hippocampus. J. Physiol. (Lond.) 471, 245–268 (1993).
Waters, J. & Smith, S. J. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J. Neurosci. 20, 7863–7870 (2000).
Chazot, P. L. & Stephenson, F. A. Biochemical evidence for the existence of a pool of unassembled C2 exon-containing NR1 subunits of the mammalian forebrain NMDA receptor. J. Neurochem. 68, 507–516 (1997).
Okabe, S., Miwa, A. & Okado, H. Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. J. Neurosci. 19, 7781–7792 (1999).
Lu, W. et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29, 243–254 (2001).
Heynen, A. J., Quinlan, E. M., Bae, D. C. & Bear, M. F. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 28, 527–536 (2000).
Shi, S., Hayashi, Y., Esteban, J. A. & Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001).
Standley, S., Roche, K. W., McCallum, J., Sans, N. & Wenthold, R. J. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron 28, 887–898 (2000).
Vissel, B., Krupp, J. J., Heinemann, S. F. & Westbrook, G. L. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat. Neurosci. 4, 587–596 (2001).
Roche, K. W. et al. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 4, 794–802 (2001).
Lu, Y. M., Roder, J. C., Davidow, J. & Salter, M. W. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279, 1363–1367 (1998).
Salter, M. W. Src, N-methyl-D-aspartate (NMDA) receptors, and synaptic plasticity. Biochem. Pharmacol. 56, 789–798 (1998).
Gardoni, F. et al. Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J. Neurosci. 21, 1501–1509 (2001).
Benke, T. A., Lüthi, A., Isaac, J. T. & Collingridge, G. L. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393, 793–797 (1998).
Derkach, V., Barria, A. & Soderling, T. R. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA 96, 3269–3274 (1999).
Lee, H. K., Barbarosie, M., Kameyama, K., Bear, M. F. & Huganir, R. L. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 (2000).
Snell, L. D. et al. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res. Mol. Brain Res. 40, 71–78 (1996).
Stone, L. M., Browning, M. D. & Finger, T. E. Differential distribution of the synapsins in the rat olfactory bulb. J. Neurosci. 14, 301–309 (1994).
Wenthold, R. J., Yokotani, N., Doi, K. & Wada, K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J. Biol. Chem. 267, 501–507 (1992).
Petralia, R. S., Wang, Y. X., Mayat, E. & Wenthold, R. J. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J. Comp. Neurol. 385, 456–476 (1997).
Porter, R. M. et al. Monoclonal antibodies to cytoskeletal proteins: an immunohistochemical investigation of human colon cancer. J. Pathol. 170, 435–440 (1993).
Siegel, S. J. et al. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc. Natl. Acad. Sci. USA 91, 564–568 (1994).
Acknowledgements
We thank M.L. Dell'Acqua, J.F. MacDonald and A.C. Spalding for advice concerning this manuscript. D.R.G. and D.A.C. are funded by individual NRSAs from NIMH. S.J.C. is supported by an individual NRSA from NIAAA. This work was also funded by NIAAA RO1 AA09675 and NIH AG04418-17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.D.B. has a financial interest in Phosphoresolutions (Aurora, Colorado), a company that sells some of the NMDA antibodies used in this study.
Rights and permissions
About this article
Cite this article
Grosshans, D., Clayton, D., Coultrap, S. et al. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci 5, 27–33 (2002). https://doi.org/10.1038/nn779
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn779
This article is cited by
-
Nanoscale rules governing the organization of glutamate receptors in spine synapses are subunit specific
Nature Communications (2022)
-
Long-term potentiation prevents ketamine-induced aberrant neurophysiological dynamics in the hippocampus-prefrontal cortex pathway in vivo
Scientific Reports (2020)
-
Maternal Gastrointestinal Nematode Infection Up-regulates Expression of Genes Associated with Long-Term Potentiation in Perinatal Brains of Uninfected Developing Pups
Scientific Reports (2019)
-
Postsynaptic RIM1 modulates synaptic function by facilitating membrane delivery of recycling NMDARs in hippocampal neurons
Nature Communications (2018)
-
Oxytocin and vasopressin modulation of social anxiety following adolescent intermittent ethanol exposure
Psychopharmacology (2018)