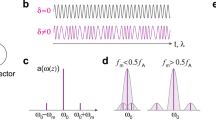
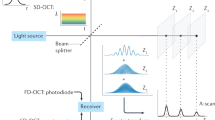
Abstract
Optical elastography, the use of optics to characterize and map the mechanical properties of biological tissue, involves measuring the deformation of tissue in response to a load. Such measurements may be used to form an image of a mechanical property, often elastic modulus, with the resulting mechanical contrast complementary to the more familiar optical contrast. Optical elastography is experiencing new impetus in response to developments in the closely related fields of cell mechanics and medical imaging, aided by advances in photonics technology, and through probing the microscale between that of cells and whole tissues. Two techniques — optical coherence elastography and Brillouin microscopy — have recently shown particular promise for medical applications, such as in ophthalmology and oncology, and as new techniques in cell mechanics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cowin, S. C. & Doty, S. B. Tissue Mechanics (Springer, 2007).
Janmey, P. A. & McCulloch, C. A. Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 9, 1–34 (2007).
Parker, K. J., Doyley, M. M. & Rubens, D. J. Imaging the elastic properties of tissue: the 20 year perspective. Phys. Med. Biol. 56, R1–R29 (2011).
Jaffer, O. S., Lung, P. F. C., Bosanac, D., Shah, A. & Sidhu, P. S. Is ultrasound elastography of the liver ready to replace biopsy? A critical review of the current techniques. Ultrasound 20, 24–32 (2012).
Wojcinski, S. et al. Multicenter study of ultrasound real-time tissue elastography in 779 cases for the assessment of breast lesions: improved diagnostic performance by combining the BI-RADS®-US classification system with sonoelastography. Ultraschall Med. 31, 484–491 (2010).
Lee, G. Y. & Lim, C. T. Biomechanics approaches to studying human diseases. Trends Biotechnol. 25, 111–118 (2007).
Kennedy, B. F., Kennedy, K. M. & Sampson, D. D. A review of optical coherence elastography: fundamentals, techniques and prospects. IEEE J. Sel. Top. Quant. Electron. 20, 272–288 (2014).
Wang, S. & Larin, K. V. Optical coherence elastography for tissue characterization: a review. J. Biophoton. 8, 279–302 (2015).
Larin, K. V. & Sampson, D. D. Optical coherence elastography — OCT at work in tissue biomechanics. Biomed. Opt. Express 8, 1172–1202 (2017).
Schmitt, J. M. OCT elastography: imaging microscopic deformation and strain of tissue. Opt. Express 3, 199–211 (1998).
Scarcelli, G. & Yun, S. H. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat. Photon. 2, 39–43 (2008).
Jacques, S. L. & Kirkpatrick, S. J. Acoustically modulated speckle imaging of biological tissues. Opt. Lett. 23, 879–881 (1998).
Bossy, E. et al. Transient optoelastography in optically diffusive media. Appl. Phys. Lett. 90, 174111 (2007).
Mohan, K. D. & Oldenburg, A. L. Elastography of soft materials and tissues by holographic imaging of surface acoustic waves. Opt. Express 20, 18887–18897 (2012).
Von Gierke, H. E., Oestreicher, H. L., Franke, E. K., Parrack, H. O. & von Wittern, W. W. Physics of vibrations in living tissues. J. Appl. Physiol. 4, 886–900 (1952).
Orr, J. F. & Shelton, J. C. Optical Measurement Methods in Biomechanics (Chapman & Hall, 1997).
Leitgeb, R., Hitzenberger, C. & Fercher, A. Performance of Fourier domain vs. time domain optical coherence tomography. Opt. Express 11, 889–894 (2003).
Antonacci, G. et al. Quantification of plaque stiffness by Brillouin microscopy in experimental thin cap fibroatheroma. J. R. Soc. Interface 12, 20150843 (2015).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Kennedy, B. F. et al. Investigation of optical coherence microelastography as a method to visualize cancers in human breast tissue. Cancer Res. 75, 3236–3245 (2015).
Allen, W. M. et al. Wide-field optical coherence micro-elastography for intraoperative assessment of human breast cancer margins. Biomed. Opt. Express 7, 4139–4153 (2016).
Kennedy, B. F., Malheiro, F. G., Chin, L. & Sampson, D. D. Three-dimensional optical coherence elastography by phase-sensitive comparison of C-scans. J. Biomed. Opt. 19, 076006 (2014).
Kennedy, K. M. et al. Quantitative micro-elastography: imaging of tissue elasticity using compression optical coherence elastography. Sci. Rep. 5, 15538 (2015).
Qi, W. et al. Resonant acoustic radiation force optical coherence elastography. Appl. Phys. Lett. 103, 103704 (2013).
Akca, B. I. et al. Observation of sound-induced corneal vibrational modes by optical coherence tomography. Biomed. Opt. Express 6, 3313–3319 (2015).
Adie, S. G. et al. Spectroscopic optical coherence elastography. Opt. Express 18, 25519–25534 (2010).
Sarvazyan, A. P., Rudenko, O. V., Swanson, S. D., Fowlkes, J. B. & Emelianov, S. Y. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 24, 1419–1435 (1998).
Wang, S. & Larin, K. V. Noncontact depth-resolved micro-scale optical coherence elastography of the cornea. Biomed. Opt. Express 5, 3807–3821 (2014).
Song, S., Le, N. H., Huang, Z., Shen, T. & Wang, R. K. Quantitative shear-wave optical coherence elastography with a programmable phased array ultrasound as the wave source. Opt. Lett. 40, 5007–5010 (2015).
Li, C., Guan, G., Cheng, X., Huang, Z. & Wang, R. K. Quantitative elastography provided by surface acoustic waves measured by phase-sensitive optical coherence tomography. Opt. Lett. 37, 722–724 (2012).
Singh, M. et al. Phase-sensitive optical coherence elastography at 1.5 million A-lines per second. Opt. Lett. 40, 2588–2591 (2015).
Leroux, C.-E., Palmier, J., Boccara, A. C., Cappello, G. & Monnier, S. Elastography of multicellular aggregates submitted to osmo-mechanical stress. N. J. Phys. 17, 073035 (2015).
Nahas, A., Bauer, M., Roux, S. & Boccara, A. C. 3D static elastography at the micrometer scale using full field OCT. Biomed. Opt. Express 4, 2138–2149 (2013).
Zaitsev, V. Y., Matveev, L. A., Matveyev, A. L., Gelikonov, G. V. & Gelikonov, V. M. Elastographic mapping in optical coherence tomography using an unconventional approach based on correlation stability. J. Biomed. Opt. 19, 021107 (2014).
Wang, R. K., Ma, Z. & Kirkpatrick, S. J. Tissue Doppler optical coherence elastography for real time strain rate and strain mapping of soft tissue. Appl. Phys. Lett. 89, 144103 (2006).
Kennedy, B. F. et al. Optical coherence micro-elastography: mechanical-contrast imaging of tissue microstructure. Biomed. Opt. Express 5, 2113–2124 (2014).
Bouwens, A. et al. Quantitative lateral and axial flow imaging with optical coherence microscopy and tomography. Opt. Express 21, 17711–17729 (2013).
Antonacci, G., Foreman, M. R., Paterson, C. & Torok, P. Spectral broadening in Brillouin imaging. Appl. Phys. Lett. 103, 221105 (2013).
Scarcelli, G. et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat. Methods 12, 1132–1136 (2015).
Scarcelli, G., Kim, P. & Yun, S. H. In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys. J. 101, 1539–1545 (2011).
Scarcelli, G. et al. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest. Ophthalmol. Vis. Sci. 54, 1418–1425 (2013).
Scarcelli, G., Besner, S., Pineda, R., Kalout, P. & Yun, S. H. In vivo biomechanical mapping of normal and keratoconus corneas. JAMA Ophthalmol. 133, 480–482 (2015).
Ballmann, C. W. et al. Stimulated Brillouin scattering microscopic imaging. Sci. Rep. 5, 18139 (2015).
Li, C. et al. Detection and characterisation of biopsy tissue using quantitative optical coherence elastography (OCE) in men with suspected prostate cancer. Cancer Lett. 357, 121–128 (2015).
Liang, X., Oldenburg, A. L., Crecea, V., Chaney, E. J. & Boppart, S. A. Optical micro-scale mapping of dynamic biomechanical tissue properties. Opt. Express 16, 11052–11065 (2008).
Ford, M. R., Roy, A. S., Rollins, A. M. & Dupps, W. J. Serial biomechanical comparison of edematous, normal, and collagen crosslinked human donor corneas using optical coherence elastography. J. Cataract Refr. Surg. 40, 1041–1047 (2014).
Curatolo, A. et al. Ultrahigh resolution optical coherence elastography. Opt. Lett. 41, 21–24 (2015).
Crecea, V., Graf, B. W., Taewoo, K., Popescu, G. & Boppart, S. A. High resolution phase-sensitive magnetomotive optical coherence microscopy for tracking magnetic microbeads and cellular mechanics. IEEE J. Sel. Top. Quant. Electron. 20, 6800907 (2014).
Hajjarian, Z. & Nadkarni, S. K. Evaluating the viscoelastic properties of tissue from laser speckle flucuations. Sci. Rep. 2, 1–8 (2012).
Kennedy, K. M., Kennedy, B. F., McLaughlin, R. A. & Sampson, D. D. Needle optical coherence elastography for tissue boundary detection. Opt. Lett. 37, 2310–2312 (2012).
Dong, L. et al. Quantitative compression optical coherence elastography as an inverse elasticity problem. IEEE J. Sel. Top. Quant. Electron. 22, 6802211 (2016).
Mulligan, J. A., Untracht, G. R., Chandrasekaran, S. N., Brown, C. N. & Adie, S. G. Emerging approaches for high-resolution imaging of tissue biomechanics with optical coherence elastography. IEEE J. Sel. Top. Quant. Electron. 22, 6800520 (2016).
Acknowledgements
The authors thank their colleagues past and present who have contributed to the evolution of optical elastography; in particular, S. Adie, W. Allen, L. Chin, B. Quirk, A. Curatolo, S. Es'hagian, K. Kennedy, R. Kirk, R. McLaughlin and P. Munro. This work has been supported in part by the Australian Research Council, the National Health and Medical Research Council, the National Breast Cancer Foundation, and the Western Australian Department of Health. P.W. thanks the Schrader Trust for a studentship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
OncoRes Medical Pty Ltd has recently been established to develop optical elastography for applications in breast-conserving surgery. B.F.K. and D.D.S. have shares in OncoRes Medical and in future B.F.K. will be undertaking funded research for this company.
Rights and permissions
About this article
Cite this article
Kennedy, B., Wijesinghe, P. & Sampson, D. The emergence of optical elastography in biomedicine. Nature Photon 11, 215–221 (2017). https://doi.org/10.1038/nphoton.2017.6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2017.6
This article is cited by
-
Brillouin microscopy
Nature Reviews Methods Primers (2024)
-
Mechanical experimentation of the gastrointestinal tract: a systematic review
Biomechanics and Modeling in Mechanobiology (2024)
-
Segmenting mechanically heterogeneous domains via unsupervised learning
Biomechanics and Modeling in Mechanobiology (2024)
-
Ultra-wideband optical coherence elastography from acoustic to ultrasonic frequencies
Nature Communications (2023)
-
Pulsed stimulated Brillouin microscopy enables high-sensitivity mechanical imaging of live and fragile biological specimens
Nature Methods (2023)