Abstract
The field of metabolomics continues to catalog new compounds, but their functional analysis remains technically challenging, and roles beyond metabolism are largely unknown. Unbiased genetic/RNAi screens are powerful tools to identify the in vivo functions of protein-encoding genes, but not of nonproteinaceous compounds such as lipids. They can, however, identify the biosynthetic enzymes of these compounds—findings that are usually dismissed, as these typically synthesize multiple products. Here, we provide a method using follow-on biosynthetic pathway screens to identify the endpoint biosynthetic enzyme and thus the compound through which they act. The approach is based on the principle that all subsequently identified downstream biosynthetic enzymes contribute to the synthesis of at least this one end product. We describe how to systematically target lipid biosynthetic pathways; optimize targeting conditions; take advantage of pathway branchpoints; and validate results by genetic assays and biochemical analyses. This approach extends the power of unbiased genetic/RNAi screens to identify in vivo functions of non-nucleic acid–based metabolites beyond their metabolic roles. It will typically require several months to identify a metabolic end product by biosynthetic pathway screens, but this time will vary widely depending, among other factors, on the end product's location in the pathway, which determines the number of screens required for its identification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
27 May 2015
In the version of this article initially published, the text detailing the HPLC elution protocol in the Equipment Setup section (a 2-min column pre-equilibration in 9:1 A:B (vol/vol); sample injection; 2-min wash with 100% A; 14-min linear gradient to 100% B; and 15-min post-run column equilibration) was incorrect and has been changed to read: a 2-min column pre-equilibration in 9:1 A:B (vol/vol); sample injection; 2-min wash with 100% A; 12-min linear gradient to 100% B; and 1-min post-run column equilibration. The error has been corrected in the HTML and PDF versions of the article.
References
van Meer, G., Voelker, D.R. & Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Blom, T., Somerharju, P. & Ikonen, E. Synthesis and biosynthetic trafficking of membrane lipids. Cold Spring Harb Perspect Biol. 3, a004713 (2011).
Wenk, M.R. Lipidomics: new tools and applications. Cell 143, 888–895 (2010).
Ivanova, P.T., Milne, S.B., Myers, D.S. & Brown, H.A. Lipidomics: a mass spectrometry based systems level analysis of cellular lipids. Curr. Opin. Chem. Biol. 13, 526–531 (2009).
Halabalaki, M., Vougogiannopoulou, K., Mikros, E. & Skaltsounis, A.L. Recent advances and new strategies in the NMR-based identification of natural products. Curr. Opin. Biotechnol. 25C, 1–7 (2014).
Jungmann, J.H. & Heeren, R.M. Emerging technologies in mass spectrometry imaging. J. Proteomics. 75, 5077–5092 (2012).
Zhang, H. et al. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat. Cell Biol. 13, 1189–1201 (2011).
Breslow, D.K. & Weissman, J.S. Membranes in balance: mechanisms of sphingolipid homeostasis. Mol. Cell 40, 267–279 (2010).
Hannun, Y.A. & Obeid, L.M. Many ceramides. J. Biol. Chem. 286, 27855–27862 (2011).
Watts, J.L. & Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99, 5854–5859 (2002).
Ashrafi, K. et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421, 268–272 (2003).
Kniazeva, M., Crawford, Q.T., Seiber, M., Wang, C.Y. & Han, M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PloS Biol. 2, E257 (2004).
Brock, T.J., Browse, J. & Watts, J.L. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2, e108 (2006).
Entchev, E.V. et al. LET-767 is required for the production of branched chain and long chain fatty acids in Caenorhabditis elegans. J. Biol. Chem. 283, 17550–17560 (2008).
Elle, I.C., Olsen, L.C., Pultz, D., Rodkaer, S.V. & Faergeman, N.J. Something worth dyeing for: molecular tools for the dissection of lipid metabolism in Caenorhabditis elegans. FEBS Lett. 584, 2183–2193 (2010).
Barros, A.G., Liu, J., Lemieux, G.A., Mullaney, B.C. & Ashrafi, K. Analyses of C. elegans fat metabolic pathways. Methods Cell Biol. 107, 383–407 (2012).
Papan, C. et al. Systematic screening for novel lipids by shotgun lipidomics. Anal. Chem. 86, 2703–2710 (2014).
Vance, D.E. & Vance, J.E. Biochemistry of Lipids, Lipoproteins and Membranes 5th edn.(Elsevier, 2008).
Xu, Y.H., Barnes, S., Sun, Y. & Grabowski, G.A. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J. Lipid Res. 51, 1643–1675 (2010).
Kniazeva, M., Euler, T. & Han, M. A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans. Genes Dev. 22, 2102–2110 (2008).
Shi, Y. & Burn, P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat. Rev. Drug Discov. 3, 695–710 (2004).
Hess, D., Chisholm, J.W. & Igal, R.A. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS ONE 5, e11394 (2010).
Kamphorst, J.J. et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 110, 8882–8887 (2013).
Delgado, A., Casas, J., Llebaria, A., Abad, J.L. & Fabrias, G. Inhibitors of sphingolipid metabolism enzymes. Biochim. Biophys. Acta 1758, 1957–1977 (2006).
Albi, E. & Viola Magni, M. Sphingolipid metabolism inhibitors and cell function. Open Enzyme Inhib. J. 1, 72–79 (2008).
Ohba, Y. et al. Mitochondria-type GPAT is required for mitochondrial fusion. EMBO J. 32, 1265–1279 (2013).
Marza, E., Simonsen, K.T., Faergeman, N.J. & Lesa, G.M. Expression of ceramide glucosyltransferases, which are essential for glycosphingolipid synthesis, is only required in a small subset of C. elegans cells. J. Cell Sci. 122, 822–833 (2009).
Zhu, H., Shen, H., Sewell, A.K., Kniazeva, M. & Han, M. A novel sphingolipid-TORC1 pathway critically promotes postembryonic development in Caenorhabditis elegans. Elife 2, e00429 (2013).
Krank, J., Murphy, R.C., Barkley, R.M., Duchoslav, E. & McAnoy, A. Qualitative analysis and quantitative assessment of changes in neutral glycerol lipid molecular species within cells. Methods Enzymol. 432, 1–20 (2007).
Ivanova, P.T., Milne, S.B., Byrne, M.O., Xiang, Y. & Brown, H.A. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 432, 21–57 (2007).
Deems, R., Buczynski, M.W., Bowers-Gentry, R., Harkewicz, R. & Dennis, E.A. Detection and quantitation of eicosanoids via high-performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 432, 59–82 (2007).
Sullards, M.C. et al. Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: 'inside-out' sphingolipidomics. Methods Enzymol. 432, 83–115 (2007).
Garrett, T.A., Guan, Z. & Raetz, C.R. Analysis of ubiquinones, dolichols, and dolichol diphosphate-oligosaccharides by liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 432, 117–143 (2007).
McDonald, J.G., Thompson, B.M., McCrum, E.C. & Russell, D.W. Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Methods Enzymol. 432, 145–170 (2007).
Deline, M.L., Vrablik, T.L. & Watts, J.L. Dietary supplementation of polyunsaturated fatty acids in Caenorhabditis elegans. J. Vis. Exp. 81, e50879 (2013).
Yook, K. Complementation. WormBook ed. The C. elegans Research Community) http://dx.doi.org/10.1895/wormbook.1.24.1 (2005).
Acknowledgements
C. elegans strains were provided by G. Ruvkun (Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA), S. Mitani (National Bioresource Project Japan) and the Caenorhabditis Genetics Center (NIH Center for Research Resources). We thank J. Moore (Avanti Polar Lipids) and E. Membreno for contributions to LC/MS and C. elegans maintenance, respectively. We thank H. Weinstein and R. Kleinman for ongoing support. This work was supported by US National Institutes of Health grant GM078653, Massachusetts General Hospital IS Funding and a Mattina R. Proctor Award to V.G.
Author information
Authors and Affiliations
Contributions
H.Z. performed most experiments, carried out tier I and II screens, organized the manuscript and wrote its technical part. N.A. participated in most experiments and executed tier III screens. L.A.K. helped with genetic interaction experiments. V.G. conceived the idea, directed the project, participated in experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
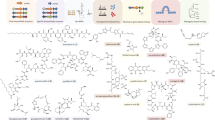
Supplementary Figure 1 Examples of chemical structures for the 8 lipid categories.
Categories a - f originate entirely or in part from the biochemical subunit of a ketoacyl group (A) and categories g - h from that of an isoprene group (B). Schematics are drawn using ChemDraw software. Fatty acids, made of a hydrocarbon chain that terminates with a carboxylic acid group, are building-blocks for glycerolipids, glycerophospholipids, sphingolipids and saccharolipids. Glycerolipids, the major storage fat in animal tissues, are typically composed of fatty acid mono-, di- and tri-esters of glycerol and are components of glycerophospholipids, also referred to as phospholipids. Phospholipids, components of membranes and signaling molecules, are mainly derived from sn-1,2-diacylglycerols with a phosphate residue in position sn-3 linked to an amino-alcohol, amino acid or another moiety. Sphingolipids, likewise components of membranes and signaling molecules, contain a sphingoid base backbone that is synthesized from the amino acid serine and a long-chain fatty acyl CoA; this long chain base forms ceramide through the addition of another long-chain fatty acyl CoA, and is further converted into phosphosphingolipids and other complex sphingolipids. Saccharolipids are components of bacterial membranes and define a class of compounds in which fatty acids are linked directly to a sugar backbone, thereby forming membrane bilayer compatible structures. Polyketides, a collection of secondary metabolites of various biological activities made by many organisms, refer to cyclic molecules, synthesized through the elongation of malonyl-CoA-derived extender units in a similar process to FA synthesis, followed by hydrolysis- or cyclization-induced termination. Sterols, membrane components and hormone precursors in animal cells, and prenols (vitamins, ubiquinones, and important components of posttranslational protein modification), are predominantly synthesized by the classical mevalonate pathway. Precursors fuse by successive addition of C5 units to form the four-ring core structure of steroids or to form prenol lipids.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 558 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Abraham, N., Khan, L. et al. RNAi-based biosynthetic pathway screens to identify in vivo functions of non-nucleic acid–based metabolites such as lipids. Nat Protoc 10, 681–700 (2015). https://doi.org/10.1038/nprot.2015.031
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.031
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.