Key Points
-
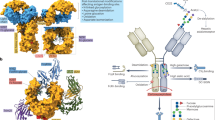
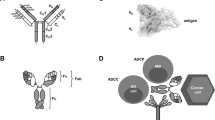
Natural and recombinant immunoglobulin G (IgG) antibodies consist of an IgG Fab (antigen-binding fragment) region and IgG Fc (crystallizable fragment) regions. On antigen binding, antibodies form multimeric complexes that can initiate inflammatory processes leading to their elimination and destruction.
-
Glycosylation of the IgG Fc region is essential for the activation of downstream effector functions. There is heterogeneity in the oligosaccharides that are attached, resulting in multiple glycoforms that can differ from each other in biological efficacy.
-
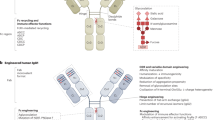
Production cell lines may be engineered to produce selected glycoforms with enhanced biological properties and to provide maximum efficacy for a given disease indication.
-
It may be possible to further improve the effector function of currently approved therapeutic antibodies, without increasing their immunogenicity, by generating particular glycoforms.
-
Improved production vehicles can offer lower cost of goods and contribute to lower cost of treatment.
Abstract
To date, more than 20 recombinant immunoglobulin G (IgG) antibody therapeutics are licensed for the treatment of various diseases. The mechanism of action of recombinant monoclonal antibodies (rMAbs) has been extensively investigated and several distinct pathways have been defined; selective activation of specific pathways may optimize clinical outcomes for different diseases, such as cancer and chronic inflammation. Human IgG is a glycoprotein with oligosaccharides attached at a single site. These are essential to the mode of action of rMAbs, and the antibody efficacy can vary depending on the particular oligosaccharide that is attached. Methods are now becoming available that allow the production of rMAbs bearing pre-selected oligosaccharides — glycoforms — to provide maximum efficacy for a given disease indication. This Review summarizes current knowledge of these methods and avenues for their exploitation in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Moutel, S. & Perez, F. Antibodies — Europe. Engineering the next generation of antibodies. Biotechnol. J. 3, 298–300 (2008).
Reichert, J. M. & Valge-Archer, V. E. Development trends for monoclonal antibody cancer therapeutics. Nature Rev. Drug Discov. 6, 349–356 (2007).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Mirik, G. R., Bradt, B. M., Denardo, S. J. & Denardo, G. L. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q. J. Nucl. Med. Mol. Imaging 48, 251–257 (2004).
Koren, E. et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J. Immunol. Methods 333, 1–9 (2008).
Aarden, L., Ruul, S. R. & Wolbink, G. Immunogenicity of anti-tumor necrosis factor antibodies-toward improved methods of anti-antibody measurement. Curr. Opin. Immunol. 20, 431–435 (2008).
Klitgaard, J.L. et al. Reduced susceptibility of recombinant polyclonal antibodies to inhibitory anti-variable domain antibody responses. J. Immunol. 177, 3782–3790 (2006).
Woof, J. M. & Burton, D.R. Human antibody–Fc receptor interactions illuminated by crystal structures. Nature Rev. Immunol. 4, 89–99 (2004).
Nezlin, R. & Ghetie, V. Interactions of immunoglobulins outside the antigen-combining site. Adv. Immunol. 82, 155–215 (2004).
Jefferis, R. Antibody therapeutics: isotype and glycoform selection. Expert Opin. Biol. Ther. 7, 1401–1413 (2007).
van Sorge, N. M., van der Pol, W. L. & van de Winkel, J. G. FcγR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens 61, 189–202 (2003).
Nimmerjahn, F. & Ravetch, J. Fcγ receptors as regulators of immune responses. Nature Rev. Immunol. 8, 34–47 (2008).
Jefferis, R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 21, 11–16 (2005).
Sinclair, A. M. & Elliott, S. J. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J. Pharm. Sci. 94, 1626–1635 (2005).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003).
Niwa, R. et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods 306, 151–160 (2005). A demonstration that non-fucosylated glycoforms of all four IgG subclasses can bind and activate FcγRIIIa.
Ferrara, C. et al. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous β1,4-N-acetylglucosaminyltransferase III and Golgi α-mannosidase II. Biotechnol. Bioeng. 93, 851–861 (2006).
Krapp, S. et al. Structural analysis of human IgG glycoforms reveals a correlation between oligosaccharide content, structural integrity and Fcγ-receptor affinity. J. Mol. Biol. 325, 979–989 (2003).
Deisenhofer, J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry 20, 2361–2370 (1981). This early paper showed that oligosaccharides are integral to an antibody's three-dimensional structure.
Arnold, J. N., Wormald, M. R., Sim, R. B., Rudd, P. M. & Dwek, R. A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 25, 21–50 (2007).
Mizuochi, T., Taniguchi, T. Shimizu, A. & Kobata, A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J. Immunol. 129, 2016–2020 (1982).
Routier, F. H. et al. Quantitation of the oligosaccharides of human serum IgG from patients with rheumatoid arthritis: a critical evaluation of different methods. J. Immunol. Methods 213, 13–30 (1998).
Kaneko, Y., Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006).
Scallon, B., Tam, S. H., McCarthy, S. G., Cai, A. N. & Raju, T. S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 44, 1524–1534 (2007).
Nimmerjahn, F. & Ravetch, J. V. The antiinflammatory activity of IgG: the intravenous IgG paradox. J. Exp. Med. 204, 11–15 (2007).
Anthony, R. M. et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 (2008).
Jefferis, R. et al. A comparative study of the N-linked oligosaccharide structures of human IgG subclass proteins. Biochem. J. 268, 529–537 (1990).
Farooq, M., Takahashi N., Arrol, H., Drayson, M. & Jefferis, R. Glycosylation of polyclonal and paraprotein IgG in multiple myeloma. Glycoconjugate J. 14, 489–492 (1997).
Youings, A. et al. Site-specific glycosylation of human immunoglobulin G is altered in four rheumatoid arthritis patients. Biochem. J. 314, 621–630 (1996).
Holland, M. et al. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA associated systemic vasculitis. Biochim. Biophys. Acta 1760, 669–677 (2006).
Mimura, Y., Ashton, P. R., Takahashi, N., Harvey, D. J. & Jefferis, R. Contrasting glycosylation profiles between Fab and Fc of a human IgG protein studied by electrospray ionization mass spectrometry. J. Immunol. Methods 326, 116–126 (2007).
Mimura, Y. et al. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol. Immunol. 37, 697–706 (2000).
Birch, J. R. & Racher, A. J. Antibody production. Adv. Drug Deliv. Rev. 58, 671–685 (2006).
Imai-Nishiya, H. et al. Double knockdown of α1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 7, 84 (2007).
Macher, B. A. & Galili, U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta 1780, 75–88 (2008).
Padler-Karavani, V. et al. Diversity in specificity, abundance and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology 18, 818–830 (2008).
Qian, J. et al. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal. Biochem. 364, 8–18 (2007).
Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N. Engl. J. Med. 358, 1109–1117 (2008). Important report of hypersensitivity reactions to a recombinant therapeutic due to production in Sp2/0 cells in which gal a (1–3) gal is added.
Huang, L., Biolosi, S., Bales, K. R. & Kuchibhotla, U. Impact of variable domain glycosylation on antibody clearance: an LC/MS characterization. Anal. Biochem. 349, 197–207 (2006).
Carter, P. et al., Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992).
Lim, A., Reed-Bogan, A. & Harmon, B. J. Glycosylation profiling of a therapeutic recombinant monoclonal antibody with two N-linked glycosylation sites using liquid chromatography coupled to a hybrid quadrupole time-of-flight mass spectrometer. Anal. Biochem. 375, 163–172 (2008).
Desjarlais, J. R., Lazar, G. A., Zhukovsky E. A. & Chu, S. Y. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer's perspective. Drug Discov. Today 12, 898–910 (2007).
Sondermann, P., Huber, R., Oosthuizen, V. & Jacob, U. The 3.2-Å crystal structure of the human IgG1 Fc–FcγRIII complex. Nature 406, 267–273 (2000). First structural confirmation of the interaction site of IgG Fc for an Fcγ receptor. This has allowed in silico modelling of other FcγRs.
Radaev, S. et al. The structure of human type FcγIII receptor in complex with Fc. J. Biol. Chem. 276, 16469–16477 (2001).
Mimura, Y. et al. The role of oligosaccharide residues of IgG1-Fc in FcγIIb binding. J. Biol. Chem. 276, 5539–45547 (2001).
Matsumiya, S. et al. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J. Mol. Biol. 368, 767–779 (2007).
Watier, H. Variability factors in the clinical response to recombinant antibodies and IgG Fc-containing fusion proteins. Expert Opin. Biol. Ther. 5 (Suppl. 1), S1–S8 (2005).
Marcus, R. & Hagenbeek, A. The therapeutic use of rituximab in non-Hodgkin's lymphoma. Eur. J. Haematol. 78 (Suppl. 67), 5–14 (2007).
Lifely, M. R., Hale, G. & Boyse, S. Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology 5, 813–822 (1995).
Umana, P. et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nature Biotechnol. 17, 176–180 (1999). First report of a cell line glycoengineered to produce a recombinant antibody with enhanced efficacy for killing cancer cells.
Davies, J. et al. Expression of GTIII in a recombinant anti-CD20 CHO production cell line: expression of antibodies of altered glycoforms leads to an increase in ADCC thro' higher affinity for FcRIII. Biotechnol. Bioeng. 74, 288–294 (2001).
Ymane-Ohuki, N. et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 87, 614–622 (2004).
Iida, S. et al. Non-fucosylated therapeutic IgG antibody can evade the inhibitory effect of serum IgG on antibody-dependent cellular cytotoxicity through its binding to FcγRIIIa. Clin. Cancer Res. 12, 2879–2887 (2006).
Preithner, S. et al. High concentrations of therapeutic IgG1 antibodies are needed to compensate for the inhibition of ADCC by excess endogenous immunoglobulin G. Mol. Immunol. 43, 1183–1193 (2006).
Nechansky, A. et al. Compensation of endogenous IgG mediated inhibition of antibody-dependent cellular cytotoxicity by glyco-engineering of therapeutic antibodies. Mol. Immunol. 44, 1815–1817 (2007).
Suzuki, E. et al. Nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin. Cancer Res. 13, 1875–1882 (2007).
Peipp, M. et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood 112, 2390–2399 (2008). Potentially important finding that optimal IgG Fc glycoforms for cellular activation may differ between PBMCs and PMNs.
Ferarra, C., Stuart, F., Sondermann, P., Brunker, P. & Umana, P. The carbohydrate at FcγaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281, 5032–5036 (2006).
Boyd, P. N., Lines, A. C. & Patel, A. K. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol. Immunol. 32, 1311–1318 (1995).
US Food and Drug Administration (FDA). Memorandum. FDA web site [online], (1997).
Malhotra, R. et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nature Med. 1, 237–243 (1995).
Abadeh, S. et al. Remodelling the oligosaccharide of human IgG antibodies: effects on biological activities. Biochem. Soc. Trans. 25, S661 (1997).
Arnold, J. N., Dwek, R. A., Rudd, P. M. & Sim, R. B. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol. Lett. 106, 103–110 (2006).
Garred, P. et al. J. Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J. Rheumatol. 27, 26–34 (2000).
Sato, R. et al. Substances reactive with mannose-binding protein (MBP) in sera of patients with rheumatoid arthritis. Fukushima J. Med. Sci. 43, 99–111 (1997).
Saevarsdottir, S., Vikingsdottir, T. & Valdimarsson, H. The potential role of mannan-binding lectin in the clearance of self-components including immune complexes. Scand. J. Immunol. 60, 23–29 (2004).
Taylor, M. E. & Drickamer, K. Paradigms for glycan-binding receptors in cell adhesion. Curr. Opin. Cell Biol. 19, 572–577 (2007).
Vyas, J. M., Van der Veen, A. G., & Ploegh, H. L. The known unknowns of antigen processing and presentation. Nature Rev. Immunol. 8, 607–618 (2008).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nature Rev. Immunol. 7, 715–725 (2007).
Harris, R. J. Heterogeneity of recombinant antibodies: linking structure to function. Dev. Biol. (Basel) 122, 117–127 (2005).
Jones, A. J. et al. Selective clearance of glycoforms of a complex glycoprotein pharmaceutical caused by terminal N-acetylglucosamine is similar in humans and cynomolgus monkeys. Glycobiology 17, 529–540 (2007).
Keck, R. et al. Characterization of a complex glycoprotein whose variable metabolic clearance in humans is dependent on terminal N-acetylglucosamine content. Biologicals 36, 49–60 (2008).
Edberg, J. C. & Kimberly, R. P. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand binding. J. Immunol. 159, 3849–3857 (1997).
Watt, G. et al. Synthesis of homogeneous neoglycoforms of IgG-Fc molecules and their functional properties. Hum. Antibodies 11, 29–30 (2002).
Zhou, Q. et al. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol. Bioeng. 99, 652–665 (2008).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Roy Jefferis has consultancy contracts with Novartis, Merck and Gilde.
Glossary
- Diantennary oligosaccharide
-
An oligosaccharide that has two mannose 'arms'; as opposed to triantennary, tetra-antennary and so on.
- Differential scanning micro-calorimetry
-
A thermoanalytic technique in which the difference in the amount of heat required to increase the temperature of a sample and a reference is measured as a function of temperature.
- Alanine scanning
-
A genetic manipulation that sequentially replaces wild-type amino acids with alanine to determine the impact on the protein's structure and function.
- Conformer
-
A discrete, defined conformation in three-dimensional space.
- Antibody-dependent cellular cytotoxicity
-
(ADCC). Cell death that results when the Fc fragment of an antibody bound to a target cell interacts with Fc receptors on monocytes, macrophages or natural killer cells that are consequently activated to kill the target.
- Complement-dependent cytotoxicity
-
(CDC). Cell death that results when the IgG Fc regions of an antibody bound to a target cell activate the C1 component of complement, initiating a cascade of reactions that lead to the formation of a complex that disrupts the cell membrane.
- Granzyme
-
An apoptosis-inducing serine protease released from cytoplasmic granules by cytotoxic T cells and NK cells.
Rights and permissions
About this article
Cite this article
Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 8, 226–234 (2009). https://doi.org/10.1038/nrd2804
Issue Date:
DOI: https://doi.org/10.1038/nrd2804
This article is cited by
-
Synthesis of N-acetylglucosamine analogues modified at C6 position with azido-derived moieties
Monatshefte für Chemie - Chemical Monthly (2024)
-
CE-MS/MS and CE-timsTOF to separate and characterize intramolecular disulfide bridges of monoclonal antibody subunits and their application for the assessment of subunit reduction protocols
Analytical and Bioanalytical Chemistry (2024)
-
Altered glycosylation profiles of serum IgG in Takayasu arteritis
European Journal of Medical Research (2023)
-
Emerging phagocytosis checkpoints in cancer immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Rapid biosynthesis of glycoprotein therapeutics and vaccines from freeze-dried bacterial cell lysates
Nature Protocols (2023)