Key Points
-
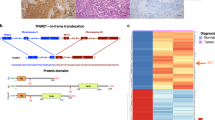
The Pax-8–PPAR-γ fusion protein (PPFP) is a consequence of a chromosomal translocation found in approximately one-third of follicular carcinomas and some follicular-variant papillary carcinomas and benign follicular adenomas
-
In vitro and in vivo evidence indicates that PPFP acts as an oncoprotein
-
PPFP can act as a dominant-negative inhibitor of wild-type PPAR-γ and/or as a PPAR-γ-like transcription factor, and can activate or repress Pax-8-responsive genes
-
The PPAR-γ agonist pioglitazone has beneficial effects in a mouse model of PPFP-positive thyroid carcinoma, and is currently being tested in a phase II clinical trial
Abstract
Thyroid carcinoma is the most common endocrine malignancy, and its incidence is continuing to increase. Most thyroid carcinomas contain one of several known driver mutations, such as the Val600Glu substitution in B-Raf, Ras mutations, RET gene fusions, or PAX8–PPARG gene fusions. The PAX8–PPARG gene fusion results in the production of a Pax-8–PPAR-γ fusion protein (PPFP), which is found in approximately one-third of follicular thyroid carcinomas, as well as some follicular-variant papillary thyroid carcinomas. In vitro and in vivo evidence indicates that PPFP is an oncoprotein. Although specific mechanisms of action remain to be defined, PPFP is considered to act as a dominant-negative inhibitor of wild-type PPAR-γ and/or as a unique transcriptional activator of subsets of PPAR-γ-responsive and Pax-8-responsive genes. Detection of the fusion transcript in thyroid nodule biopsy specimens can aid clinical decision-making when cytological findings are indeterminate. The PPAR-γ agonist pioglitazone is highly therapeutic in a transgenic mouse model of PPFP-positive thyroid carcinoma, suggesting that PPAR-γ agonists might be beneficial in patients with PPFP-positive thyroid carcinomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
American Cancer Society. Cancer Facts and Figures 2014 [online] (2014).
Enewold, L. et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol. Biomarkers Prev. 18, 784–791 (2009).
Wiest, P. W. et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J. Ultrasound Med. 17, 487–496 (1998).
Tomimori, E., Pedrinola, F., Cavaliere, H., Knobel, M. & Medeiros-Neto, G. Prevalence of incidental thyroid disease in a relatively low iodine intake area. Thyroid 5, 273–276 (1995).
Greaves, T. S. et al. Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer 90, 335–341 (2000).
Sclabas, G. M. et al. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am. J. Surg. 186, 702–710 (2003).
Papini, E. et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J. Clin. Endocrinol. Metab. 87, 1941–1946 (2002).
Carroll, B. A. Asymptomatic thyroid nodules: incidental sonographic detection. AJR Am. J. Roentgenol. 138, 499–501 (1982).
Yassa, L. et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 111, 508–516 (2007).
Brander, A., Viikinkoski, P., Nickels, J. & Kivisaari, L. Thyroid gland: US screening in a random adult population. Radiology 181, 683–687 (1991).
Mazzaferri, E. L. Thyroid cancer in thyroid nodules: finding a needle in the haystack. Am. J. Med. 93, 359–362 (1992).
Frates, M. C. et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J. Clin. Endocrinol. Metab. 91, 3411–3417 (2006).
Namba, H. et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 88, 4393–4397 (2003).
Kim, K. H., Kang, D. W., Kim, S. H., Seong, I. O. & Kang, D. Y. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med. J. 45, 818–821 (2004).
Cohen, Y. et al. BRAF mutation in papillary thyroid carcinoma. J. Natl Cancer Inst. 95, 625–627 (2003).
Adeniran, A. J. et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am. J. Surg. Pathol. 30, 216–222 (2006).
Trovisco, V. et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J. Pathol. 202, 247–251 (2004).
Rabes, H. M. et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res. 6, 1093–1103 (2000).
Nikiforov, Y. E., Rowland, J. M., Bove, K. E., Monforte-Munoz, H. & Fagin, J. A. Distinct pattern of RET oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 57, 1690–1694 (1997).
Bounacer, A. et al. High prevalence of activating RET proto-oncogene rearrangements, in thyroid tumors from patients who had received external radiation. Oncogene 15, 1263–1273 (1997).
Leeman-Neill, R. J. et al. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer 119, 1792–1799 (2013).
Esapa, C. T., Johnson, S. J., Kendall-Taylor, P., Lennard, T. W. & Harris, P. E. Prevalence of Ras mutations in thyroid neoplasia. Clin. Endocrinol. (Oxf.) 50, 529–535 (1999).
Motoi, N. et al. Role of ras mutation in the progression of thyroid carcinoma of follicular epithelial origin. Pathol. Res. Pract. 196, 1–7 (2000).
Namba, H., Rubin, S. A. & Fagin, J. A. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol. Endocrinol. 4, 1474–1479 (1990).
French, C. A. et al. Genetic and biological subgroups of low-stage follicular thyroid cancer. Am. J. Pathol. 162, 1053–1060 (2003).
Nikiforova, M. N. et al. RAS point mutations and PAX8–PPARγ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 88, 2318–2326 (2003).
Marques, A. R. et al. Expression of PAX8–PPARγ 1 rearrangements in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 87, 3947–3952 (2002).
Castro, P. et al. PAX8–PPARγ rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 91, 213–220 (2006).
Nikiforova, M. N., Biddinger, P. W., Caudill, C. M., Kroll, T. G. & Nikiforov, Y. E. PAX8–PPARγ rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am. J. Surg. Pathol. 26, 1016–1023 (2002).
Dwight, T. et al. Involvement of the PAX8/peroxisome proliferator-activated receptor γ rearrangement in follicular thyroid tumors. J. Clin. Endocrinol. Metab. 88, 4440–4445 (2003).
Lui, W.-O. et al. CREB3L2–PPARγ fusion mutation identifies a thyroid signaling pathway regulated by intramembrane proteolysis. Cancer Res. 68, 7156–7164 (2008).
Fagin, J. A. et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J. Clin. Invest. 91, 179–184 (1993).
Donghi, R. et al. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J. Clin. Invest. 91, 1753–1760 (1993).
Dobashi, Y. et al. Stepwise participation of p53 gene mutation during dedifferentiation of human thyroid carcinomas. Diagn. Mol. Pathol. 3, 9–14 (1994).
Ito, T. et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 52, 1369–1371 (1992).
Garcia-Rostan, G. et al. β-Catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am. J. Pathol. 158, 987–996 (2001).
Garcia-Rostan, G. et al. Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res. 59, 1811–1815 (1999).
Kurihara, T. et al. Immunohistochemical and sequencing analyses of the Wnt signaling components in Japanese anaplastic thyroid cancers. Thyroid 14, 1020–1029 (2004).
Ricarte-Filho, J. C. et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 69, 4885–4893 (2009).
Garcia-Rostan, G. et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 65, 10199–10207 (2005).
Santarpia, L., El-Naggar, A. K., Cote, G. J., Myers, J. N. & Sherman, S. I. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 93, 278–284 (2008).
Hou, P. et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin. Cancer Res. 13, 1161–1170 (2007).
Dahia, P. L. et al. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 57, 4710–4713 (1997).
Kim, C. S. et al. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology 146, 4456–4463 (2005).
Saito, J. et al. Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid 11, 339–351 (2001).
Pasca di Magliano, M., Di Lauro, R. & Zannini, M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl Acad. Sci. USA 97, 13144–13149 (2000).
Macchia, P. E. et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat. Genet. 19, 83–86 (1998).
Poleev, A. et al. Determination of functional domains of the human transcription factor PAX8 responsible for its nuclear localization and transactivating potential. Eur. J. Biochem. 247, 860–869 (1997).
Poleev, A. et al. Distinct functional properties of three human paired-box-protein, PAX8, isoforms generated by alternative splicing in thyroid, kidney and Wilms tumors. Eur. J. Biochem. 228, 899–911 (1995).
Rosen, E. D. et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 (1999).
Yamauchi, T. et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 276, 41245–41254 (2001).
Xu, H. E. et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell 3, 397–403 (1999).
Forman, B. M. et al. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83, 803–812 (1995).
Kliewer, S. A. et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl Acad. Sci. USA 94, 4318–4323 (1997).
Raman, P., Kaplan, B. L. F., Thompson, J. T., Vanden Heuvel, J. P. & Kaminski, N. E. 15-Deoxy-Δ12,14-prostaglandin J2-glycerol ester, a putative metabolite of 2-arachidonyl glycerol, activates peroxisome proliferator activated receptor γ. Mol. Pharmacol. 80, 201–209 (2011).
Raman, P., Kaplan, B. L. F. & Kaminski, N. E. 15-Deoxy-Δ12,14-prostaglandin J2-glycerol, a putative metabolite of 2-arachidonyl glycerol and a peroxisome proliferator-activated receptor γ ligand, modulates nuclear factor of activated T cells. J. Pharmacol. Exp. Ther. 342, 816–826 (2012).
Corzo, C. & Griffin, P. R. Targeting the peroxisome proliferator-activated receptor-γ to counter the inflammatory milieu in obesity. Diabetes Metab. J. 37, 395–403 (2013).
Mueller, E. et al. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor γ isoforms. J. Biol. Chem. 277, 41925–41930 (2002).
Fajas, L. et al. The organization, promoter analysis, and expression of the human PPARγ gene. J. Biol. Chem. 272, 18779–18789 (1997).
Tontonoz, P., Hu, E., Graves, R. A., Budavari, A. I. & Spiegelman, B. M. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 (1994).
Kroll, T. G. et al. PAX8–PPARγ1 fusion oncogene in human thyroid carcinoma [corrected]. Science 289, 1357–1360 (2000).
Mascia, A., Nitsch, L., Di Lauro, R. & Zannini, M. Hormonal control of the transcription factor Pax8 and its role in the regulation of thyroglobulin gene expression in thyroid cells. J. Endocrinol. 172, 163–176 (2002).
Cheung, L. et al. Detection of the PAX8–PPAR γ fusion oncogene in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 88, 354–357 (2003).
Martelli, M. L. et al. Inhibitory effects of peroxisome proliferator-activated receptor γ on thyroid carcinoma cell growth. J. Clin. Endocrinol. Metab. 87, 4728–4735 (2002).
Zhu, Z., Gandhi, M., Nikiforova, M. N., Fischer, A. H. & Nikiforov, Y. E. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of Ras mutations. Am. J. Clin. Pathol. 120, 71–77 (2003).
Aldred, M. A. et al. Peroxisome proliferator-activated receptor γ is frequently downregulated in a diversity of sporadic nonmedullary thyroid carcinomas. Oncogene 22, 3412–3416 (2003).
Lacroix, L. et al. PAX8 and peroxisome proliferator-activated receptor γ 1 gene expression status in benign and malignant thyroid tissues. Eur. J. Endocrinol. 151, 367–374 (2004).
Hibi, Y. et al. Is thyroid follicular cancer in Japanese caused by a specific t(2;3)(q13;p25) translocation generating Pax8–PPARγ fusion mRNA? Endocr. J. 51, 361–366 (2004).
Marques, A. R. et al. Underexpression of peroxisome proliferator-activated receptor (PPAR)γ in PAX8/PPARγ-negative thyroid tumours. Br. J. Cancer 91, 732–738 (2004).
Sahin, M. et al. PPARγ staining as a surrogate for PAX8/PPARγ fusion oncogene expression in follicular neoplasms: clinicopathological correlation and histopathological diagnostic value. J. Clin. Endocrinol. Metab. 90, 463–468 (2005).
Castro, P., Roque, L., Magalhães, J. & Sobrinho-Simões, M. A subset of the follicular variant of papillary thyroid carcinoma harbors the PAX8–PPARγ translocation. Int. J. Surg. Pathol. 13, 235–238 (2005).
Foukakis, T. et al. The Ras effector NORE1A is suppressed in follicular thyroid carcinomas with a PAX8–PPARγ fusion. J. Clin. Endocrinol. Metab. 91, 1143–1149 (2006).
Giordano, T. J. et al. Delineation, functional validation, and bioinformatic evaluation of gene expression in thyroid follicular carcinomas with the PAX8–PPARG translocation. Clin. Cancer Res. 12, 1983–1993 (2006).
Klemke, M. et al. On the prevalence of the PAX8–PPARG fusion resulting from the chromosomal translocation t(2;3)(q13;p25) in adenomas of the thyroid. Cancer Genet. 204, 334–339 (2011).
Jenkins, R. B. et al. Frequent occurrence of cytogenetic abnormalities in sporadic nonmedullary thyroid carcinoma. Cancer 66, 1213–1220 (1990).
Boos, L. A. et al. Diagnostic and prognostic implications of the PAX8–PPARγ translocation in thyroid carcinomas—a TMA-based study of 226 cases. Histopathology 63, 234–241 (2013).
Gregory Powell, J. et al. The PAX8/PPARγ fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARγ inhibition. Oncogene 23, 3634–3641 (2004).
Au, A. Y. M. et al. PAX8–peroxisome proliferator-activated receptor γ (PPARγ) disrupts normal PAX8 or PPARγ transcriptional function and stimulates follicular thyroid cell growth. Endocrinology 147, 367–376 (2006).
Vu-Phan, D. et al. The thyroid cancer PAX8–PPARG fusion protein activates Wnt/TCF-responsive cells that have a transformed phenotype. Endocr. Relat. Cancer 20, 725–739 (2013).
Aiello, A. et al. Peroxisomal proliferator-activated receptor-γ agonists induce partial reversion of epithelial–mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology 147, 4463–4475 (2006).
Ohta, K., Endo, T. & Onaya, T. The mRNA levels of thyrotropin receptor, thyroglobulin and thyroid peroxidase in neoplastic human thyroid tissues. Biochem. Biophys. Res. Commun. 174, 1148–1153 (1991).
Park, J.-W. et al. Troglitazone, the peroxisome proliferator-activated receptor-γ agonist, induces antiproliferation and redifferentiation in human thyroid cancer cell lines. Thyroid 15, 222–231 (2005).
Kitamura, S. et al. Peroxisome proliferator-activated receptor γ induces growth arrest and differentiation markers of human colon cancer cells. Jpn J. Cancer Res. 90, 75–80 (1999).
Keelan, J. A. et al. 15-Deoxy-Δ12,14-prostaglandin J2, a ligand for peroxisome proliferator-activated receptor-γ, induces apoptosis in JEG3 choriocarcinoma cells. Biochem. Biophys. Res. Commun. 262, 579–585 (1999).
Takahashi, N. et al. Activation of PPARγ inhibits cell growth and induces apoptosis in human gastric cancer cells. FEBS Lett. 455, 135–139 (1999).
Tontonoz, P. et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proc. Natl Acad. Sci. USA 94, 237–241 (1997).
Kubota, T. et al. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res. 58, 3344–3352 (1998).
Elstner, E. et al. Ligands for peroxisome proliferator-activated receptor γ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl Acad. Sci. USA 95, 8806–8811 (1998).
Kato, Y. et al. PPARγ insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-κB signaling pathway. Oncogene 25, 2736–2747 (2006).
Karger, S. et al. Evaluation of peroxisome proliferator-activated receptor-γ expression in benign and malignant thyroid pathologies. Thyroid 15, 997–1003 (2005).
Lacroix, L. et al. Follicular thyroid tumors with the PAX8–PPARγ1 rearrangement display characteristic genetic alterations. Am. J. Pathol. 167, 223–231 (2005).
Wood, W. M. et al. PPARγ promotes growth and invasion of thyroid cancer cells. PPAR Res. 2011, 171765 (2011).
Burton, J. D., Goldenberg, D. M. & Blumenthal, R. D. Potential of peroxisome proliferator-activated receptor γ antagonist compounds as therapeutic agents for a wide range of cancer types. PPAR Res. 2008, 494161 (2008).
Dobson, M. E. et al. Pioglitazone induces a proadipogenic antitumor response in mice with PAX8–PPARγ fusion protein thyroid carcinoma. Endocrinology 152, 4455–4465 (2011).
Diallo-Krou, E. et al. Paired box gene 8–peroxisome proliferator-activated receptor-γ fusion protein and loss of phosphatase and tensin homolog synergistically cause thyroid hyperplasia in transgenic mice. Endocrinology 150, 5181–5190 (2009).
Reddi, H. V. et al. Expression of the PAX8/PPARγ fusion protein is associated with decreased neovascularization in vivo: impact on tumorigenesis and disease prognosis. Genes Cancer 1, 480–492 (2010).
Albelda, S. M., Oliver, P. D., Romer, L. H. & Buck, C. A. EndoCAM: a novel endothelial cell–cell adhesion molecule. J. Cell Biol. 110, 1227–1237 (1990).
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
Espadinha, C., Cavaco, B. M. & Leite, V. PAX8PPARγ stimulates cell viability and modulates expression of thyroid-specific genes in a human thyroid cell line. Thyroid 17, 497–509 (2007).
Robson, E. J., He, S.-J. & Eccles, M. R. A PANorama of PAX genes in cancer and development. Nat. Rev. Cancer 6, 52–62 (2006).
Lui, W.-O. et al. Expression profiling reveals a distinct transcription signature in follicular thyroid carcinomas with a PAX8–PPARγ fusion oncogene. Oncogene 24, 1467–1476 (2005).
Albuquerque, C. et al. Colorectal cancers show distinct mutation spectra in members of the canonical WNT signaling pathway according to their anatomical location and type of genetic instability. Genes Chromosomes Cancer 49, 746–759 (2010).
Armstrong, M. J. et al. PAX8/PPARγ rearrangement in thyroid nodules predicts follicular-pattern carcinomas, in particular the encapsulated follicular variant of papillary carcinoma. Thyroid http://dx.doi.org/10.1089/thy.2014.0067.
Lehmann, J. M. et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J. Biol. Chem. 270, 12953–12956 (1995).
US National Library of Medicine. ClinicalTrails.gov [online], (2012).
Acknowledgements
R.J.K.'s research work was supported by NIH grants R01CA151842 and R01CA166033.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Raman, P., Koenig, R. Pax-8–PPAR-γ fusion protein in thyroid carcinoma. Nat Rev Endocrinol 10, 616–623 (2014). https://doi.org/10.1038/nrendo.2014.115
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.115
This article is cited by
-
Genomic alterations in thyroid cancer: biological and clinical insights
Nature Reviews Endocrinology (2024)
-
Pathogenesis of cancers derived from thyroid follicular cells
Nature Reviews Cancer (2023)
-
Novel thrombospondin-1 transcript exhibits distinctive expression and activity in thyroid tumorigenesis
Oncogene (2023)
-
B cells and tertiary lymphoid structures are associated with survival in papillary thyroid cancer
Journal of Endocrinological Investigation (2023)
-
Overview of the 2022 WHO Classification of Thyroid Neoplasms
Endocrine Pathology (2022)