Key Points
-
Epicardial adipose tissue is the fat depot between the myocardium and the visceral layer of the pericardium, and is anatomically and functionally contiguous with the myocardium
-
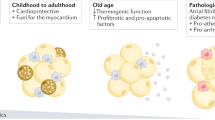
This tissue has the greatest rates of lipogenesis and fatty acid metabolism among visceral fat depots and displays metabolic, thermogenic (similar to brown fat) and mechanical (cardioprotective) properties
-
A transcriptome unique to epicardial adipose is enriched in genes associated with inflammation, endothelial function coagulation and regulation of potassium channels
-
The myocardium is modulated by cytokines, which are secreted by the epicardial fat depot
-
Epicardial fat is a marker of visceral adiposity and can be used to test the efficacy of interventions aimed at modulating adipose tissue characteristics
-
Coronary artery disease, the metabolic syndrome, insulin resistance, fatty liver disease and cardiac abnormalities are associated with increased amounts of epicardial fat
Abstract
Epicardial adipose tissue is a unique and multifaceted fat depot with local and systemic effects. This tissue is distinguished from other visceral fat depots by a number of anatomical and metabolic features, such as increased fatty acid metabolism and a unique transcriptome enriched in genes that are associated with inflammation and endothelial function. Epicardial fat and the heart share an unobstructed microcirculation, which suggests these tissues might interact. Under normal physiological conditions, epicardial fat has metabolic, thermogenic (similar to brown fat) and mechanical (cardioprotective) characteristics. Development of pathological conditions might drive the phenotype of epicardial fat such that it becomes harmful to the myocardium and the coronary arteries. The equilibrium between protective and detrimental effects of this tissue is fragile. Expression of the epicardial-fat-specific transcriptome is downregulated in the presence of severe and advanced coronary artery disease. Improved local vascularization, weight loss and targeted medications can restore the protective physiological functions of epicardial fat. Measurements of epicardial fat have several important applications in the clinical setting: accurate measurement of its thickness or volume is correlated with visceral adiposity, coronary artery disease, the metabolic syndrome, fatty liver disease and cardiac changes. On account of this simple clinical assessment, epicardial fat is a reliable marker of cardiovascular risk and an appealing surrogate for assessing the efficacy of drugs that modulate adipose tissues.
Similar content being viewed by others
Introduction
Interest in organ-specific adiposity is rapidly growing as a substantial accumulation of scientific-based evidence indicates that anatomic specificity is an important contributor to the pathophysiology of cardiometabolic and endocrine diseases. In this context, epicardial fat, which is the visceral fat of the heart, has emerged as an adipose depot of interest to scientists and physicians who focus on stratification of cardiometabolic risk factors. This interest is largely due to the key localization of this fat depot, along with its peculiar metabolic properties and clinical measurability. The attention from the scientific community has brought this previously neglected fat depot into the spotlight with almost 800 peer-reviewed articles published in the past decade. This Review provides an updated overview of basic research studies and clinical applications of epicardial fat measurements.
Anatomy
Macroscopic
Epicardial adipose tissue is the fat depot situated between the myocardium and the visceral layer of the pericardium (Figure 1).1 This depot is present in humans and other large mammals, but only minimally present or completely absent in rats or mice.2 Epicardial fat originates from the splanchnopleuric mesoderm and is vascularized by branches of the coronary arteries.2 Distinguishing epicardial from pericardial adipose tissue is important, as the two fat depots are embryologically, anatomically and functionally distinct.3 The pericardial fat depot is situated outside the visceral pericardium and on the external surface of the parietal pericardium, originates from the primitive thoracic mesenchyme and is vascularized by noncoronary arteries.1 Epicardial fat is located within the heart and is commonly found in the atrioventricular and interventricular grooves, but can also expand from the epicardial surface into the myocardium.4 Remarkably, no fascia (as found on skeletal muscle) separates this fat from the underlying myocardium.1 Epicardial fat can also be located directly within the myocardium or around the coronary artery adventitia.5 This contiguity with the adventitia and the absence of muscle fascia suggest that paracrine or vasocrine crosstalk between the epicardial fat and the myocardium occurs. Although epicardial fat and the myocardium share the coronary blood supply, as yet, a direct microcirculatory interconnection between the two tissues has not been proven. However, strong evidence of a microcirculatory connection between epicardial fat and the coronary wall via vasa vasora exists.1,2,4
Epicardial adipose tissue is defined as the fat located between the myocardium and visceral pericardium; whereas pericardial adipose tissue is the fat depot located outside the visceral pericardium and on the external surface of the parietal pericardium. Epicardial fat is the fat depot immediately adjacent to the heart and pericardial fat is the outer fat depot of the heart.
Microscopic
Epicardial adipose tissue is composed of mainly adipocytes, but also contains stromovascular and immune cells, as well as ganglia and interconnecting nerves.6 By comparison with cells of other visceral fat depots, including pericardial fat, epicardial adipocytes are generally smaller, possibly as a result of their peculiar anatomical location, the greater number of preadipocytes than mature adipocytes in this depot, and a high energy-consuming metabolism that might prevent large amounts of lipid storage.7 This metabolic phenotype might also explain why epicardial fat is poorly represented in animals with a fast metabolism, such as mice. Generally, epicardial fat is considered to be a white adipose tissue. However, new findings suggest that epicardial adipocytes might have features that closely resemble brown or beige adipocytes.8 In fact, small unilocular adipocytes that are positive for mitochondrial brown fat uncoupling protein 1 (UCP1) have been described in epicardial fat, which suggests that these cells share some histological features with beige and brown adipocytes.8
Physiology
Owing to its contiguity with the myocardium, epicardial fat has unique physiological functions. The roles of epicardial fat within the heart are complex, but can be grossly distinguished by metabolic, thermogenic and mechanical functions.9,10
Metabolism
Epicardial fat is rich in saturated fatty acids and has a high protein content, as well as having the greatest capacity for free fatty acid (FFA) release and uptake compared with other visceral fat depots.11,12 Under physiological conditions, this enrichment and increased metabolism of FFAs is functionally important to the myocardium, as energy production in the heart is mainly generated by FFA oxidation.10,11 The myocardium metabolizes FFAs present in the coronary arterial bloodstream, which is shared with the contiguous epicardial fat. FFAs might diffuse bidirectionally through the interstitial fluid across concentration gradients from epicardial fat into the myocardium. The influx of FFAs into the coronary artery is facilitated by fatty-acid-binding protein 4 (also known as fatty acid-binding protein, adipocyte) and vasoactive cytokines, which are secreted by the epicardial fat to regulate coronary arterial tone.13 This mechanism ensures that the myocardium is buffered from exposure to excessively high levels of FFAs by the epicardial fat.
Thermogenesis
The potential thermogenic actions of epicardial fat are newly described and are receiving increasing attention.10 Epicardial fat has been suggested to function similarly to brown adipose tissue (BAT) and to provide direct heat to the myocardium.14 Epicardial adipose tissue might, therefore, function to protect the heart during a drop in core body temperature or during unfavourable haemodynamic conditions such as ischaemia or hypoxia. This working concept has been formed on the basis of the high level of expression of BAT-specific genes, such as UCP1, PRDM16 and PPARGC1A, in human epicardial fat.14 In particular, expression of UCP1 is considerably higher in epicardial fat than in other fat depots, and is essentially undetectable in subcutaneous fat.14 Interestingly UCP1 expression in epicardial fat is also associated with high circulating levels of HDL cholesterol.15 The hypothesis that epicardial fat provides heat to the myocardium is also supported by the presence of a large fat pad that covers the heart in hibernating animals.2 Whether epicardial fat is a BAT or functions as a BAT-like depot is unclear and remains the subject of discussion.8 Moreover, although human epicardial fat expresses abundant levels of UCP1 protein,14 which can mediate thermogenesis in mature beige cells, the myocardium might receive sufficient heat from splitting ATP molecules during each cardiac contraction.16
Mechanical functions
In addition to the important metabolic and thermogenic properties, epicardial fat functions mechanically to protect the coronary artery against the torsion that is induced by the arterial pulse wave and cardiac contraction.17 In this role, the intrinsic compressibility of the epicardial fat permits expansion and positive remodelling of coronary vessels.
Physiopathology
The equilibrium between the physiological and pathophysiological properties of epicardial fat is delicate and susceptible to the influences of intrinsic and external factors. In addition to its unique anatomical location, the intense metabolic activity of epicardial fat can be a major contributor to disruption of this balance. In fact, epicardial fat is the source of a number of bioactive cytokines that can either protect or adversely affect the myocardium and coronary arteries.1 The human epicardial fat secretosome is large and diverse, which probably reflects the complex cellularity and myriad of properties of epicardial fat. Furthermore, the transcriptome of epicardial fat is markedly different from that of subcutaneous fat, with the majority of relatively enriched genes being associated with endothelial function, coagulation and inflammation.6
Epicardial fat expresses and secretes proinflammatory and anti-inflammatory adipokines, as well as vasoactive factors and growth factors that, given the absence of anatomical barriers, can signal to the myocardium through either paracrine or autocrine pathways.18 In the case of the paracrine pathway, cytokines from the epicardial fat diffuse through the adventitia, media and intima into the lumen of the coronary artery.18,19 Alternatively, epicardial adipokines can be secreted directly into the vasa vasorum, through a vasocrine signalling pathway.19
Under normal physiological conditions epicardial fat can, therefore, exert cardioprotective actions through paracrine or vasocrine secretion of anti-atherogenic cytokines, such as adiponectin and adrenomedullin.20,21 However, if the equilibrium of the epicardial fat secretosome is disrupted, production and secretion of protective adipokines can be downregulated. For example, expression of adiponectin in epicardial adipose tissue is lower in patients with coronary artery disease (CAD) or heart failure than in healthy individuals.20,22 Epicardial adipose tissue can also be harmful, as proinflammatory and atherogenic cytokines, such as C-C motif chemokine 2 (commonly known as monocyte chemoattractant protein 1 or MCP-1), IL-1β, IL-6 and tumour necrosis factor (TNF) become upregulated and secreted in excess into the adjacent myocardium and coronary artery bloodstream.6 Whether these changes are consequent to CAD and heart failure, or if they are a cause of these diseases is unclear; however, the idea that these processes are reciprocal and bidirectional is plausible.
The hypothesis that epicardial adipose tissue secretes adipokines directly into the underlying coronary bloodstream has been investigated by three studies, with promising but controversial results.23,24,25 Left coronary artery (LCA) levels of adiponectin significantly correlated with adiponectin protein expression in epicardial adipose tissues from patients with CAD and without CAD, although this relationship was not independent of peripheral adiponectin levels.23 In another study from the same group, adrenomedullin was simultaneously collected from the LCA and the coronary sinus during coronary angiography in patients with CAD and without CAD; levels of adrenomedullin in the coronary sinus were not statistically different from those in the LCA.24 However, levels of mRNA transcripts encoding adrenomedullin in epicardial fat tissue correlated with levels of the protein in the LCA.24 By contrast, another group of researchers did not find significant differences in the blood concentrations of a number of adipokines, such as adiponectin, leptin and resistin, that were simultaneously measured in the femoral artery rather than the LCA and coronary sinus of healthy individuals.25 It should also be noted that expression of genes encoding adipokines in epicardial fat was not measured in this study.25 Moreover, these studies are methodologically complex. However, on the basis of these findings, it seems that epicardial adipose tissue acts as a paracrine or vasocrine organ by locally releasing bioactive cytokines into the adjacent interstitium of the myocardium and coronary arteries. Additional studies are necessary to elucidate the endocrine function of epicardial fat.
Pathology
Local effects
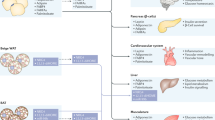
Epicardial fat elicits local effects on the function and structure of the heart. Increased amounts of epicardial fat are associated with increased left ventricular mass and abnormal right ventricle geometry.26,27 In individuals with morbid obesity, excessive epicardial fat contributes to increased left ventricular mass.26 Mechanical and biomolecular mechanisms have been suggested to explain these effects. Increased epicardial fat that results in additional mass on both ventricles can increase the work demands on the heart and lead to left ventricular hypertrophy.26 Infiltration of adipocytes from the epicardial adipose tissue to the myocardium has been also suggested to contribute to aberrant cardiac function.28 The increase in myocardial lipid content that is concurrent with increases in the amount of epicardial fat has been demonstrated by studies using proton magnetic resonance spectroscopy.29 Epicardial fat deposition can cause myocardial disarray, which can eventually lead to cardiac changes.28 Increased epicardial fat is also correlated with enlargement of the atria and impaired right and left ventricular diastolic filling.30 Physical obstruction of diastolic filling owing to presence of a large epicardial fat pad seems to be the most likely cause of these effects.29 In addition, the effects of epicardial fat on atrial dimensions might have electrophysiological consequences; the contribution of the peri-atrial epicardial fat to the development of atrial fibrillation has been explored.31,32 Epicardial fat might cause electromechanical changes in atrial tissues via local secretion of cytokines, such as TNF and IL-6, as well as FFAs.33,34
Systemic effects
Given that epicardial fat can affect the coronary arteries, this tissue can also have systemic effects and can contribute to the development and progression of CAD. A state of imbalance between cardioprotective and harmful adipokines secreted by epicardial fat is strongly linked to the development of coronary atherosclerosis in humans.35 Epicardial fat might alter the coronary arteries through multiple pathways, including macrophage activation, oxidative stress, innate inflammatory response and plaque destabilization.6 Regardless of the route, inflammatory cells secreted by epicardial fat that surrounds the adventitia might stimulate proliferation of the vasa vasorum and elicit intramural changes.18 Epicardial adipocytes have intrinsic proinflammatory and atherogenic secretion profiles. A dense inflammatory infiltrate, mainly comprising macrophages, is commonly present in epicardial fat tissue of patients with CAD.6 Interestingly, the ratio of proinflammatory M1 macrophages and anti-inflammatory M2 macrophages in epicardial fat is biased toward the M1 phenotype in patients with CAD.36 The presence of macrophages and mast cells in epicardial adipose tissue could also contribute to underlying instability that can lead to plaque rupture. Interestingly, coronary atherosclerotic plaques are more prominent in regions where epicardial fat deposits are more abundant.17 The atherogenic effects of epicardial fat tissue are exerted not only as a function of its anatomical vicinity to the plaque, but also by its intense proinflammatory activity. In fact, epicardial fat production of proinflammatory adipokines is considerably higher than that of the subcutaneous fat in patients with coronaropathies.37,38,39,40 Inflammatory markers that have been identified in the epicardial fat transcriptome include T-cell and macrophage markers, as well as B-cell-associated factors, such as transforming growth factor β2 (TGF-β2), as well as multiple chemokine ligands and chemokine receptors.6 The secretion of epicardial inflammatory molecules into the coronary bloodstream promotes atherogenesis, and likely occurs through vasocrine rather than paracrine pathways, as plaque accumulation tends to increase the arterial wall thickness.
The mechanisms by which epicardial fat can cause atherosclerosis are complex and not completely understood. Oxidative stress represents one such mechanism; higher levels of reactive oxygen species and lower expression of antioxidant enzymes (such as catalase), have been observed in the epicardial fat of individuals with CAD than in subcutaneous fat from the same individuals.41 Epicardial fat can affect the endothelium by inducing cell-surface expression of adhesion molecules and enhancing adhesion of monocytes to endothelial cells.42 Epicardial fat might contribute to the accumulation of lipids within atherosclerotic plaques as a result of increased secretion of group IID secretory phospholipase A2 (also known as sPLA2-II).43 The lipogenic effects of epicardial fat have also been attributed to the high content of conjugated fatty acids in this tissue.44
In addition, adhesion molecules that contribute to different phases of the atherosclerotic process, for example, MCP-1, growth-regulated α protein and C-C motif chemokine 5 (also known as RANTES), are highly expressed in epicardial fat tissue.42 The innate inflammatory response contributes to the atherogenicity of epicardial adipose tissues. Increased levels of nuclear factor-κB (NF-κB), c-jun N-terminal kinase activity and expression of toll-like receptors have been described in epicardial fat of individuals with CAD.41 Activation of toll-like receptors leads to translocation of NF-κB into the nucleus, which results in increased release of inflammatory cytokines, such as IL-1, IL-6, TNF and resistin.37 In patients with CAD, epicardial fat can influence myocardial glucose metabolism via abnormal expression of solute carrier family 2 facilitated glucose transporter member 4 (commonly known as GLUT4) and retinol-binding protein 4.41 Interestingly, levels of epicardial fat positively correlate with serum levels of leptin in patients with type 1 diabetes mellitus (T1DM).45 The inflammatory secretosome of epicardial fat might contribute to the pathogenesis of diabetes mellitus-related cardiomyopathy in patients with type 2 diabetes mellitus (T2DM).46 However, it could also be the case that in patients with diabetes mellitus, the atherogenic effects of epicardial fat might have different effects that, as the disease and the end-organ damage advance, could lead to a progressive, but partial, downregulation of its transcriptome. For example, as patients with CAD or diabetes mellitus progress toward end-stage disease, adaptive or even maladaptive mechanisms might cause changes in the transcriptional and fibrotic programmes of epicardial fat, which might eventually affect its cellular activities. The detrimental effects of epicardial fat might, therefore, vary at different stages of diabetes mellitus and CAD. However, the atherogenic action of epicardial fat is likely to be stronger in the early phases than in the late phases of these diseases.
Clinical implications
Imaging
Detection and quantification of epicardial fat certainly represent important and useful clinical applications, for which several imaging techniques have been developed.47,48,49,50,51 The thickness of epicardial fat tissue can be visualized and calculated with standard 2D echocardiographic methods (Figure 2).47,48,49 Epicardial fat is generally identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium.47 In addition to the easy accessibility and excellent reproducibility of this technique,47,48 echocardiographic epicardial fat independently reflects levels of intra-abdominal visceral fat, which is measured with MRI,48,49 and intra-myocardial lipid content, as calculated with magnetic resonance spectroscopy.29 However, echocardiographic determination of epicardial fat thickness does have some limitations, as it is a linear measurement that might not accurately reflect the epicardial fat volume. Multidetector CT or cardiac MRI can certainly provide a more accurate and volumetric measurement of epicardial fat, but are more expensive and cumbersome than echocardiographic determination.50,51 Multidetector CT is more sensitive and specific than echocardiography for measuring region-specific thickness and volume in deep epicardial fat layers such as the pericoronary fat.52,53 Regardless of the visualization technique implemented, epicardial fat measurement is regarded as an emergent tool for cardiovascular risk stratification.
Epicardial fat is identified as the relatively echo-free space (within the yellow line) between the outer wall of the myocardium and the visceral layer of pericardium. Epicardial fat thickness is usually reduced in the vicinity of the mid right ventricular free wall and increased in the distal portion of the right ventricular free wall. Owing to compression during diastole, the widest epicardial fat thickness (red line) is best measured at end-systole at the point on the free wall of the right ventricle at which the ultrasound beam is oriented in a perpendicular manner using the aortic annulus as an anatomic landmark. Abbreviations: AO, aortic root; LA, left atrium; LV, left ventricle; RV, right ventricle.
Biomarker of cardiometabolic diseases
Epicardial fat thickness has been consistently associated with the metabolic syndrome and its components;54 however, epicardial fat thickness varies considerably among populations of different ethnicities, as do overall distribution of body fat and risk of cardiovascular disease.55 This variation has prevented the establishment of general cut-off points for using epicardial fat thickness or volume to predict risk of the metabolic syndrome.55,56 However, levels of epicardial fat remain an objective marker of levels of visceral fat and a good predictor of the risk of developing the metabolic syndrome.57
The predictive and associative roles of epicardial fat, either measured as thickness or volume, to the development and progression of CAD has been extensively investigated and confirmed by several large population studies.58,59 Epicardial fat significantly correlates with the extent and severity of CAD, chest pain, unstable angina and coronary flow reserve.60,61 Moreover, epicardial fat has also been associated with fatal and nonfatal coronary events in the general population, regardless of the presence of traditional risk factors of cardiovascular disease, such as smoking, obesity, dyslipidaemia and diabetes mellitus.58,59
Interestingly, the correlation of epicardial fat with risk of CAD and developing high risk obstructive plaques is independent of obesity and/or the presence of coronary calcification.62,63,64,65,66 These findings are highly suggestive of a role for epicardial fat in promoting the early stages of atherosclerotic plaque formation and development of plaques with high-risk features, such as thin or ruptured cap, intraplaque haemorrhage or presence of a lipid-rich necrotic core. In fact, epicardial fat is associated with the presence of noncalcified plaques, which are unstable and, therefore, potentially the most dangerous type of plaque.67 The early atherogenic effects of this fat depot have been confirmed by the correlation of epicardial fat with indices of microvascular dysfunction in the absence of obstructive plaques.68 Levels of epicardial fat might be linked to development of early plaque components or noncalcified plaque burden in the underlying coronary artery. Some studies have reported a correlation between the volume of pericardial fat, rather than epicardial fat, and coronary artery calcium scores.69,70 However, pericardial fat is distinct from epicardial fat and is considered to be a paracardiac and mediastinal fat.3 Given that epicardial fat is in direct contiguity with the myocardium, a further differentiation of this fat depot into myocardial and coronary epicardial fats has been suggested.5 The contributions of regionally distributed epicardial fat depots to the development and progression of coronary atherosclerosis has also been investigated. An elegant experiment performed in a pig model of early-stage CAD showed that surgical removal of the coronary epicardial fat depot attenuated the progression of atherosclerosis.71 This finding supports an atherogenic role for the epicardial fat depot that surrounds the coronary arteries. In addition, epicardial fat has been associated with markers of subclinical atherosclerosis, such as carotid intima–media thickness and the apoB:apoA-1 ratio in symptomatic and asymptomatic adults with high risk of atherosclerosis, as well as in children.72,73,74,75
In patients with heart failure, levels of epicardial fat have been suggested to serve as a prognostic indicator for survival.76,77 Interestingly, epicardial fat, either volume or thickness, is lower in individuals with congestive heart failure than in individuals with normal systolic function.76,77 This reduction in levels of epicardial fat might reflect the overall fat mass reduction commonly observed in patients with heart failure. That the epicardial fat pad might incur fibrotic changes during chronic cardiac failure is an intriguing hypothesis.
Epicardial fat has been associated with fat accumulation in the liver, as both represent organ-specific fat depots in addition to sharing biochemical and embryological properties with intra-abdominal visceral fat.78 Increased release of FFAs and a state of intrinsic insulin resistance are the most likely mechanisms that link epicardial fat with infiltration of intrahepatic fat.28 Although epicardial fat reflects intramyocardial triglyceride content,29 its association with fatty liver is likely to be the result of systemic and multifactorial factors. Epicardial fat is associated with increased levels of serum transaminases and incidence of liver steatosis in individuals with obesity, however, these effects are independent of obesity and, instead, are related to excessive levels of visceral fat in these individuals.79 Remarkably, echocardiographic measurement of epicardial fat was the best predictor of ultrasound measured liver steatosis.80 Graded increases in levels of fasting glucose, triglycerides and alanine transaminase, as well as insulin resistance across higher tertiles of epicardial fat thickness, have been described.81,82 Interestingly, epicardial fat thickness was associated with a reduced coronary flow reserve in patients with non-alcoholic fatty liver.83
The relationship between thickness of epicardial fat and diabetes mellitus has been also evaluated, although large longitudinal studies for determining an independent predictive role of epicardial fat in patients with T2DM are lacking. Epicardial fat thickness was increased in patients with T2DM with subclinical atherosclerosis.84 Epicardial fat thickness was also higher in individuals with prediabetes mellitus than in normoglycaemic individuals.85 This association might be attributed to the correlation of epicardial fat thickness with insulin resistance as evaluated with euglycaemic hyperinsulinaemic clamp and surrogate markers of insulin resistance, as well as fasting insulin and insulin resistance as defined by HOMA.86 Moreover, cultured mesenchymal cells derived from epicardial fat tissue demonstrated insulin resistance and a reduced capacity for adipogenesis.87 Remarkably, studies have also shown that patients with T1DM have higher epicardial fat thickness than individuals without diabetes mellitus, independent of BMI, age or levels of glycated haemoglobin.45,88,89
In addition, the contribution of epicardial fat to other endocrinopathies is also under investigation. Interaction between the epicardial fat depot, the adrenal axis and the ovary has been proposed and deserves further attention.90,91,92 Clinical measurement of epicardial fat might be a helpful tool for stratifying cardiovascular risk in patients with adrenal gland diseases.
Effects of therapeutic targeting
The simplicity of quantification with imaging techniques and its fast metabolic response has enabled the use of epicardial fat measurements as a marker of the effectiveness of weight loss interventions, exercise and pharmaceutical treatments. Levels of epicardial fat decrease after consumption of a very low calorie diet or moderate aerobic exercise.93,94 Remarkably, epicardial fat thickness decreased more rapidly and substantially than other common indices of body fatness, which suggests that visceral fat loss occurs earlier than in other depots following implementation of weight-loss interventions.93 The effects of metabolic surgery on the epicardial fat depot are controversial. One study observed a considerable reduction in levels of epicardial fat after metabolic surgery,95 another reported smaller decreases in levels of epicardial fat compared with overall reductions in abdominal fat.96
Epicardial fat is also a target of pharmaceutical agents commonly used to treat dyslipidaemias, T1DM and T2DM. The actions of statins and thiazolidinediones, which have well-known effects in adipose tissues, have been assessed in the epicardial fat depot. The profile of the epicardial fat inflammatory secretosome of patients with T2DM improved following treatment with pioglitazone, as demonstrated by decreased expression of genes encoding IL-1β and other proinflammatory mediators.97 Treatment of fatty Zucker rats with rosiglitazone resulted in considerable upregulation of expression of Ppargc1a in epicardial adipocytes, which encodes a key mediator of brown fat adipogenesis. This suggests that thiazolidinediones have potential to induce thermogenesis in this fat depot.98 In patients who underwent percutaneous coronary intervention, treatment with atorvastatin resulted in better reductions in epicardial fat thickness than treatment with simvastatin and ezetimibe.99 The effects of atorvastatin on epicardial fat were also superior to those of pravastatin in postmenopausal women with hyperlipidaemia.100 No correlation was observed between lipid lowering effects and reductions in levels of epicardial fat, which suggests that atorvastatin exerts independent effects on the epicardial fat depot. Statins might function by targeting lipoprotein receptors that are overexpressed in the epicardial fat of patients with T2DM.101
Considerable reductions in epicardial fat thickness after recombinant human growth hormone (rhGH) replacement therapy has been described in both adolescents and adults with growth-hormone-deficiency syndrome.102,103 Epicardial fat thickness was substantially reduced after short-term rhGH replacement therapy; these patients also experienced improvements in their waist circumference and BMI measurements.102 In addition, epicardial fat seems to be clinically sensitive to levels of circulating thyroid hormones.105,106 Overt hypothyroidism and low levels of free T3 have been correlated with increased epicardial fat thickness.105,106 Interestingly, in patients with subclinical hypothyroidism, restoration of a euthyroid condition with levothyroxine was associated with a substantial reduction in epicardial fat thickness.104 Consistent with the potential thermogenic functions of epicardial fat, these preliminary findings might be of interest; however, additional clinical and experimental studies are required for confirmation of these associations.
Conclusions
Epicardial adipose tissue is a unique visceral fat depot that has multifaceted features with local and systemic physiological effects (Box 1) and immediate clinical implications (Box 2). Research on the epicardial fat depot has rapidly grown from infancy to an established field, but several questions remain to be answered.
The physiological role of epicardial fat and its adaption to different haemodynamic and metabolic conditions is still not completely understood. Whether and how epicardial fat might function as a BAT or an adaptive BAT-like depot needs rigorous evaluation. The potential of thyroid hormones to influence epicardial fat is also a topic that merits additional investigation.
Future clinical and experimental studies should aim to elucidate the role of epicardial adipose tissue in the development and progression of atherosclerosis. Monitoring changes in the epicardial fat transcriptome during different phases of CAD will help to better understand the dynamics of atherogenic effects of this tissue. That epicardial fat and its changes can be objectively measured makes its assessment an appealing pharmaceutical biomarker. Novel medications for the treatment of diabetes mellitus, such as glucagon-like peptide 1 analogues, have rapidly emerged in the past few years. Of note, treatment with a dipeptidyl peptidase-4 inhibitor increased the levels of PPARGC1α and uncoupling proteins in BAT of mice with diet-induced obesity.107 Future studies that assess the effects of these therapeutic interventions on the epicardial fat depot are warranted.
Review criteria
A search for original articles published between 1984 and 2014 with a focus on the past 10 years was performed in MEDLINE and PubMed. The search terms used were “epicardial fat” and “epicardial adipose tissue”. A total of 721 articles were found. However, the articles identified for this Review were restricted to English language full-text papers. The reference lists of identified articles were searched for additional relevant papers.
References
Iacobellis, G., Corradi, D. & Sharma, A. M. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2, 536–543 (2005).
Marchington, J. M. Mattacks, C. A. & Pond, C. M. Adipose tissue in the mammalian heart and pericardium; structure, foetal development and biochemical properties. Comp. Biochem. Physiol. B 94, 225–232 (1989).
Iacobellis, G. Epicardial and pericardial fat: close, but very different. Obesity 17, 625 (2009).
Corradi, D. et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 13, 313–316 (2004).
Company, J. M. et al. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. J. Appl. Physiol. 109, 1904–1912 (2010).
Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466 (2003).
Bambace, C. et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc. Pathol. 20, e153–e156 (2011).
Sacks, H. S. et al. Human epicardial fat exhibits beige features. J. Clin. Endocrinol. Metab. 98, E1448–E1455 (2013).
Iacobellis, G., Malavazos, A. E. & Corsi, M. M. Epicardial fat: from the biomolecular aspects to the clinical practice. Int. J. Biochem. Cell Biol. 43, 1651–1654 (2011).
Iacobellis, G. & Bianco, A. C. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 22, 450–457 (2011).
Marchington, J. M. & Pond, C. M. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int. J. Obes. 14, 1013–1022 (1990).
Pezeshkian, M. et al. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab. Syndr. Relat. Disord. 7, 125–131 (2009).
Vural, B. et al. Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome, Cardiovasc. Pathol. 17, 392–398 (2008).
Sacks, H. S. et al. Uncoupling protein-1 and related mRNAs in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 94, 3611–3615 (2009).
Chechi, K., Blanchard, P. G., Mathieu, P., Deshaies, Y. & Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int. J. Cardiol. 167, 2264–2270 (2013).
Barclay, C. J. & Widén, C. Efficiency of cross-bridges and mitochondria in mouse cardiac muscle. Adv. Exp. Med. Biol. 682, 267–278 (2010).
Prati, F. et al. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur. Heart J. 24, 329–336 (2003).
Sacks, H. S. & Fain, J. N. Human epicardial adipose tissue: a review. Am. Heart J. 153, 907–917 (2007).
Judkin, J. S., Eringa, E. & Stehouwer C. D. A. “Vasocrine signalling” from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365, 1817–1820 (2005).
Iacobellis, G. et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with CAD. Cytokine 29, 251–255 (2005).
Silaghi, A. et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am. J. Physiol. Endocrinol. Metab. 293, E1443–E1450 (2007).
Agra, R. M. et al. Adiponectin and p53 mRNA in epicardial and subcutaneous fat from heart failure patients. Eur. J. Clin. Invest. 44, 29–37 (2014).
Iacobellis, G. et al. Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm. Metab. Res. 41, 227–231 (2009).
Iacobellis, G. et al. Epicardial adipose tissue and intracoronary adrenomedullin levels in CAD. Horm. Metab. Res. 45, 855–860 (2009).
Sacks, H. S. & Johnson, E. Adipokine concentrations are similar in femoral artery and coronary venous sinus blood: evidence against in vivo endocrine secretion by human epicardial fat. Adipobiology 1, 51–56 (2009).
Iacobellis, G., Ribaudo, M. C., Zappaterreno, A., Iannucci, C. V. & Leonetti, F. Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol. 94, 1084–1087 (2004).
Iacobellis, G. Relation of epicardial fat thickness to right ventricular cavity size in obese subjects. Am. J. Cardiol. 104, 1601–1602 (2009).
Kankaanpää, M. et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J. Clin. Endocrinol. Metab. 91, 4689–4695 (2006).
Malavazos, A. E. et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am. J. Cardiol. 105, 1831–1835 (2010).
Iacobellis, G., Leonetti, F., Singh, N. & Sharma, A. M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 115, 272–223 (2007).
Iacobellis, G., Zaki, M. C., Garcia, D. & Willens, H. J. Epicardial fat in atrial fibrillation and heart failure. Horm. Metab. Res. 46, 587–590 (2014).
Thanassoulis, G. et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 3, 345–350 (2010).
Mazurek, T. et al. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am. J. Cardiol. 113, 1505–1508 (2014).
Lin, Y. K. et al. Heart failure epicardial fat increases atrial arrhythmogenesis. Int. J. Cardiol. 167, 1979–1983 (2013).
Shimabukuro, M. et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 33, 1077–1084 (2013).
Hirata, Y. et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue J. Am. Coll. Cardiol. 58, 248–255 (2011).
Baker, A. R. et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 13, 1 (2006).
Kremen, J. et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J. Clin. Endocrinol. Metab. 91, 4620–4627 (2006).
Cheng, K. H. et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with CAD. Int. J. Obes. (Lond.) 32, 268–274 (2008).
Fain, J. N. et al. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism 59, 1379–1386 (2010).
Salgado-Somoza, A, Teijeira-Fernández, E., Fernández, A. L., González-Juanatey, J. R. & Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 299, H202–H209 (2010).
Karastergiou, K. et al. Epicardial adipokines in obesity and CAD induce atherogenic changes in monocytes and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 30, 1340–1346 (2010).
Dutour, A. et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with CAD. J. Clin. Endocrinol. Metab. 95, 963–967 (2010).
Pezeshkian, M. & Mahtabipour, M. R. Epicardial and subcutaneous adipose tissue fatty acids profiles in diabetic and non-diabetic patients candidate for coronary artery bypass graft. Bioimpacts 3, 83–89 (2013).
Iacobellis, G., Diaz, S., Mendez, A. & Goldberg, R. Increased epicardial fat and plasma leptin in type 1 diabetes independently of obesity. Nutr. Metab. Cardiovasc. Dis. 24, 725–729 (2014).
Greulich, S. et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 126, 2324–2334 (2012).
Iacobellis, G. & Willens, H. J. Echocardiographic epicardial fat: a review of research and clinical applications. J. Am. Soc. Echocardiogr. 22, 1311–1319 (2009).
Iacobellis, G. et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obesity Res. 11, 304–310 (2003).
Iacobellis, G. et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 388, 5163–5168 (2003).
Nelson, A. J. et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J. Cardiovasc. Magn. Reson. 11, 15 (2009).
Sarin, S. et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am. J. Cardiol. 102, 767–771 (2008).
Wang, T. D. et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis 213, 279–287 (2010).
Maurovich-Horvat, P. et al. Influence of pericoronary adipose tissue on local coronary atherosclerosis as assessed by a novel MDCT volumetric method. Atherosclerosis. 219, 151–157 (2011).
Pierdomenico, S. D., Pierdomenico, A. M., Cuccurullo, F. & Iacobellis, G. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am. J. Cardiol. 15, 1234–1236 (2012).
Willens, H. J. et al. Comparison of epicardial and pericardial fat thickness assessed by echocardiography in African American and non-Hispanic white men: a pilot study. Ethn. Dis. 18, 311–316 (2008).
Salami, S. S. et al. Race and epicardial fat: the impact of anthropometric measurements, percent body fat and sex. Ethn. Dis. 23, 281–285 (2013).
Iacobellis, G., Willens, H. J., Barbaro, G. & Sharma, A. M. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 16, 887–892 (2008).
Mahabadi, A. A. et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf Recall Study. JACC Cardiovasc. Imaging 7, 909–916 (2014).
Mahabadi, A. A. et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 61, 1388–1395 (2013).
Sade, LE. et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis 204, 580–585 (2009).
Eroglu, S. et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of CAD. Nutr. Metab. Cardiovasc. Dis. 19, 211–217 (2009).
Iacobellis, G. et al. Epicardial fat thickness and CAD correlate independently of obesity. Int. J. Cardiol. 146, 452–454 (2011).
Yerramasu, A. et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 220, 223–230 (2012).
Nakanishi, K. et al. Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in non-obese subjects with coronary artery disease. Atherosclerosis 237, 353–360 (2014).
Ito, E. et al. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int. J. Cardiol. 167, 2852–2858 (2013).
Kunita E. et al. Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis 233, 447–453 (2014).
Alexopoulos, N. et al. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 210, 150–154 (2010).
Alam, M. S., Green R., de Kemp, R., Beanlands, R. S. & Chow, B. J. Epicardial adipose tissue thickness as a predictor of impaired microvascular function in patients with non-obstructive coronary artery disease. J. Nucl. Cardiol. 20, 804–812 (2013).
Ding, J. et al. The association of pericardial fat with calcified coronary plaque. Obesity 16, 1914–1919 (2008).
Rosito, G. A. et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117, 605–613 (2008).
McKenney, M. L. et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J. Cardiothorac. Surg. 9, 2 (2014).
Iacobellis, G. et al. Relation of subepicardial adipose tissue to carotid intima-media thickness in patients with human immunodeficiency virus. Am. J. Cardiol. 99, 1470–1472 (2007).
Natale, F. et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur. J. Echocardiogr. 10, 549–555 (2009).
Manco, M. et al. Epicardial fat, abdominal adiposity and insulin resistance in obese pre-pubertal and early pubertal children. Atherosclerosis 226, 490–495 (2013).
Cabrera-Rego, J. O. et al. Epicardial fat thickness correlates with carotid intima-media thickness, arterial stiffness, and cardiac geometry in children and adolescents. Pediatr. Cardiol. 35, 450–456 (2014).
Doesch, C. et al. Bioimpedance analysis parameters and epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure. Obesity (Silver Spring) 18, 2326–2332 (2010).
Iacobellis, G., Zaki, M. C., Garcia, D. & Willens, H. J. Epicardial fat in atrial fibrillation and heart failure. Horm. Metab. Res. 46, 587–590 (2014).
Iozzo, P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 34 (Suppl. 2), S371–S379 (2011).
Iacobellis, G. et al. Relation of epicardial fat and alanine aminotransferase in subjects with increased visceral fat. Obesity (Silver Spring) 16, 179–183 (2008).
Iacobellis, G., Barbarini, G., Letizia, C. & Barbaro, G. Epicardial fat thickness and nonalcoholic fatty liver disease in obese subjects. Obesity (Silver Spring) 22, 332–326 (2014).
Cikim, A. S. et al. Epicardial adipose tissue, hepatic steatosis and obesity. J. Endocrinol. Invest. 30, 459–464 (2007).
Yilmaz, Y. et al. Circulating vaspin levels and epicardial adipose tissue thickness are associated with impaired coronary flow reserve in patients with nonalcoholic fatty liver disease. Atherosclerosis 217, 125–129 (2011).
Cetin, M. et al. Relation of epicardial fat thickness with carotid intima-media thickness in patients with type 2 diabetes mellitus. Int. J. Endocrinol. 2013, 769175 (2013).
Iacobellis, G., Barbaro, G. & Gerstein, H. C. Relationship of epicardial fat thickness and fasting glucose. Int. J. Cardiol. 128, 424–426 (2008).
Iacobellis, G. & Leonetti, F. Epicardial adipose tissue and insulin resistance in obese subjects. J. Clin. Endocrinol. Metab. 90, 6300–6302 (2005).
Fernández-Trasancos, A. et al., Impaired adipogenesis and insulin resistance in epicardial fat-mesenchymal cells from patients with cardiovascular disease. J. Cell. Physiol. 229, 1722–1730 (2014).
Yazıcı, D. et al. Epicardial adipose tissue thickness in type 1 diabetic patients. Endocrine 40, 250–255 (2011).
Momesso, D. P. et al. Increased epicardial adipose tissue in type 1 diabetes is associated with central obesity and metabolic syndrome. Diabetes Res. Clin. Pract. 91, 47–53 (2011).
Iacobellis, G. et al. Epicardial fat thickness and left ventricular mass in subjects with adrenal incidentaloma. Endocrine http://dx.doi.org/10.1007/s12020-013-9902-5.
Borruel, S. et al. Global adiposity and thickness of intraperitoneal and mesenteric adipose tissue depots are increased in women with polycystic ovary syndrome (PCOS). J. Clin. Endocrinol. Metab. 98, 1254–1263 (2013).
Cakir, E. et al. Subclinical atherosclerosis and hyperandrogenemia are independent risk factors for increased epicardial fat thickness in patients with PCOS and idiopathic hirsutism. Atherosclerosis 226, 291–295 (2013).
Iacobellis, G., Singh, N., Wharton, S. & Sharma, A. M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 16, 1693–1697 (2008).
Kim, M. K. et al. Aerobic exercise training reduces epicardial fat in obese men. J. Appl. Physiol. 106, 5–11 (2009).
Willens, H. J. et al. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am. J. Cardiol. 99, 1242–1245 (2007).
Gaborit, B. et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J. Am. Coll. Cardiol. 60, 1381–1389 (2012).
Vasques, A. C. et al. Epicardial and pericardial fat in type 2 diabetes: favourable effects of biliopancreatic diversion. Obes. Surg. http://dx.doi.org/10.1007/s11695-014-1400-1.
Sacks, H. S. et al. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care 34, 730–733 (2011).
Distel, E. et al. Early induction of a brown-like phenotype by rosiglitazone in the epicardial adipose tissue of fatty Zucker rats. Biochimie 94, 1660–1667 (2012).
Park, J. H. et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J. Cardiovasc. Ultrasound 18, 121–126 (2010).
Alexopoulos, N. et al. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J. Am. Coll. Cardiol. 61, 1956–1961 (2013).
Nasarre, L. et al. Low density lipoprotein receptor-related protein 1 is upregulated in epicardial fat from type 2 diabetes mellitus patients and correlates with glucose and triglyceride plasma levels. Acta Diabetol. 51, 23–30 (2014).
Lanes, R. et al. Endothelial function, carotid artery intima-media thickness, epicardial adipose tissue, and left ventricular mass and function in growth hormone-deficient adolescents: apparent effects of growth hormone treatment on these parameters. J. Clin. Endocrinol. Metab. 90, 3978–3982 (2005).
Ferrante, E. et al. Epicardial fat thickness significantly decreases after short-term growth hormone (GH) replacement therapy in adults with GH deficiency. Nutr. Metab. Cardiovasc. Dis. 23, 459–465 (2013).
Yazıcı, D. et al. Effects of restoration of the euthyroid state on epicardial adipose tissue and carotid intima media thickness in subclinical hypothyroid patients. Endocrine http://dx.doi.org/10.1007/s12020-014-0372-1.
Kocyigit, I. et al. A low serum free triiodothyronine level is associated with epicardial adipose tissue in peritoneal dialysis patients. J. Atheroscler. Thromb. 21, 1066–1074 (2014).
Asik, M. et al. Evaluation of epicardial fat tissue thickness in patients with Hashimoto thyroiditis. Clin. Endocrinol. (Oxf.) 79, 571–576 (2013).
Shimasaki, T. et al. The dipeptidyl peptidase-4 inhibitor des-fluoro-sitagliptin regulates brown adipose tissue uncoupling protein levels in mice with diet-induced obesity. PLoS ONE 8, e63626 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11, 363–371 (2015). https://doi.org/10.1038/nrendo.2015.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.58
This article is cited by
-
Epicardial adipose tissue density predicts the presence of atrial fibrillation and its recurrence after catheter ablation: three-dimensional reconstructed image analysis
Heart and Vessels (2024)
-
Epicardial adipose tissue density is a better predictor of cardiometabolic risk in HFpEF patients: a prospective cohort study
Cardiovascular Diabetology (2023)
-
Association between epicardial adipose tissue and incident heart failure mediating by alteration of natriuretic peptide and myocardial strain
BMC Medicine (2023)
-
Concomitant aortic regurgitation predicts better left ventricular reverse remodeling after transcatheter aortic valve replacement
BMC Cardiovascular Disorders (2023)
-
Relationship between quantitative epicardial adipose tissue based on coronary computed tomography angiography and coronary slow flow
BMC Cardiovascular Disorders (2023)