Key Points
-
Abnormal functional and structural connectivity are candidate biomarkers for Alzheimer disease (AD) and other neurodegenerative diseases
-
The topography of abnormal functional and structural connectivity maps onto the clinical phenotype, and its severity correlates with clinical disease severity in AD and frontotemporal dementia
-
Structural—but, as yet, not functional—connectivity signatures of neurodegenerative diseases with a primary motor phenotype (for example, amyotrophic lateral sclerosis, Parkinson disease and Huntington disease) have been consistently identified
-
Functional connectivity signatures are related to specific molecular pathology in preclinical AD, and could serve as early disease markers
Abstract
Functional and structural connectivity measures, as assessed by means of functional and diffusion MRI, are emerging as potential intermediate biomarkers for Alzheimer disease (AD) and other disorders. This Review aims to summarize current evidence that connectivity biomarkers are associated with upstream and downstream disease processes (molecular pathology and clinical symptoms, respectively) in the major neurodegenerative diseases. The vast majority of studies have addressed functional and structural connectivity correlates of clinical phenotypes, confirming the predictable correlation with topography and disease severity in AD and frontotemporal dementia. In neurodegenerative diseases with motor symptoms, structural—but, to date, not functional—connectivity has been consistently found to be associated with clinical phenotype and disease severity. In the latest studies, the focus has moved towards the investigation of connectivity correlates of molecular pathology. Studies in cognitively healthy individuals with brain amyloidosis or genetic risk factors for AD have shown functional connectivity abnormalities in preclinical disease stages that are reminiscent of abnormalities observed in symptomatic AD. This shift in approach is promising, and may aid identification of early disease markers, establish a paradigm for other neurodegenerative disorders, shed light on the molecular neurobiology of connectivity disruption and, ultimately, clarify the pathophysiology of neurodegenerative diseases.
Similar content being viewed by others
Introduction
Neurodegenerative disorders are believed to be attributable to the deposition of abnormal toxic proteins in the brain (Figure 1).1 These proteins might activate a cascade of biochemical, metabolic, functional and structural changes that precede clinical symptoms by years, if not decades.2 Thus, clinical symptoms are just the final manifestation of a long-standing disease process and, traditionally, were the only aspects of the disease process that clinical neurologists could appreciate and study. Unfortunately, clinical symptoms are also nonspecific, in the sense that the same clinical phenotype can have different underlying molecular pathology (Figure 1). Given the extended time frame and the lack of clinical specificity, identification of biomarkers linking proteinopathies to symptoms is critical for early disease detection and better understanding of disease pathophysiology.
Functional and structural brain connectivity might act as intermediate biomarkers. Causative genetic mutations are shown by solid arrows and genetic risk factors by dotted arrows. The size of the arrow is roughly proportional to the population attributable fraction. The size of arrows linking molecular pathology to clinical phenotypes are roughly proportional to the prevalence of the clinical phenotype in the pertinent molecular pathology. Abbreviations: Aβ42, amyloid-β42; ALS, amyotrophic lateral sclerosis; APOE, apolipoprotein E; APP, amyloid precursor protein; bvFTD, behavioural variant frontotemporal dementia; DJ1, parkinson protein 7; DLB, dementia with Lewy bodies; EOAD, early-onset Alzheimer disease; FUS, fused in sarcoma; GBA, glucocerebrosidase; GRN, granulin; HP-tau, hyperphosphorylated-tau; LOAD, late-onset Alzheimer disease; LRRK2, leucine-rich repeat kinase 2; MAPT, microtubule-associated protein tau; PARK2, parkin RBR E3 ubiquitin protein ligase; PCA, posterior cortical atrophy; PD, Parkinson disease; PINK1, PTEN induced putative kinase 1; PPA, primary progressive aphasia; PS, presenilin; PSP, progressive supranuclear palsy; SNCA, α-synuclein; SOD1, superoxide dismutase 1; TARDBP/TDP-43, TAR DNA-binding protein 43; TOMM40, translocase of outer mitochondrial membrane 40 homologue; TREM2, triggering receptor expressed on myeloid cells 2; UPS, ubiquitin proteasome system; VCP, valosin containing protein.
Imaging techniques offer an invaluable tool to assess pathology in vivo. For example, brain atrophy as assessed on structural MRI is a good biomarker of downstream neurodegeneration in Alzheimer disease (AD) and frontotemporal lobar degeneration (FTLD).3,4 Brain connectivity markers are believed to represent reliable candidate biomarkers of downstream cognitive deficits and, more importantly, of upstream neuronal dysfunction and disease spread.5,6 The interest in this field is documented by the exponential growth of studies on brain connectivity in disorders in the past decade (around 2,300 papers published overall, and over 16,000 citations).
This article aims to review key findings on functional and structural connectivity, assessed with imaging techniques, as intermediate biomarkers for the main neurodegenerative disorders. Specifically, we will evaluate the evidence that connectivity biomarkers are associated downstream with clinical phenotypes and topographic brain imaging markers of neurodegeneration, and upstream with molecular determinants of neurodegeneration and susceptibility genes. After a brief description of traditional imaging methods used to assess brain connectivity in neurodegenerative diseases, we will extensively review connectivity studies in AD, frontotemporal dementia (FTD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS), progressive supranuclear palsy (PSP), and Huntington disease (HD). This section will be divided into two parts: connectivity correlates of clinical phenotypes, and connectivity correlates of molecular pathology. Dementia with Lewy bodies will not be addressed, as too few studies are available to draw any meaningful conclusions. Finally, we will briefly discuss future directions of research in the field.
Methods to assess brain connectivity
Functional connectivity
The main focus of the early era of functional imaging was on the identification of functional segregation—the localization of brain regions specialized in a specific function—as a principle of organization of the human brain. The concept of functional integration, that is, the integration of segregated areas, started with the application of statistical approaches assessing distributed brain activation changes,7,8 and was developed by Friston and colleagues with methods assessing functional and effective connectivity.9 Functional connectivity was originally defined as the “temporal correlations between remote neurophysiological events” as assessed on functional MRI (fMRI), PET or EEG.9 While these concepts were initially designed to assess task-related functional integration, with the emergence of resting-state fMRI10 the approach was applied to the investigation of task-free functional integration.
Resting-state (or 'task-free') fMRI measures the spontaneous low-frequency (<0.001–0.100 Hz) fluctuations of the blood oxygen level-dependent (BOLD) signal while the individual rests in the scanner without performing any specific task. According to Friston's definition, functional connectivity is measured by identifying temporal correlations in the low-frequency BOLD signal between brain regions.10 Seed-based and data-driven approaches are commonly used to identify brain regions that share a common time course of spontaneous fluctuations (commonly termed 'networks'). The potential value of using task-free fMRI has been demonstrated in clinical population studies,11,12,13 and has the advantage of not relying on any explicit temporal model or the choice of a specific task.
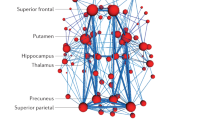
Multiple task-free networks have been consistently observed across individuals,14 sessions,15 and functional brain imaging modalities.16,17 Similar topographic mappings have been identified across multiple networks, notwithstanding the different analytical methods.18 The topography of task-free networks commonly investigated in neurodegenerative disorders is shown in Figure 2. The default mode network (DMN) is the network most consistently found in people of any age, and is also present during sleep,19 and in anaesthetized monkeys20 and rats.21 The DMN incorporates various brain regions, including the anterior and posterior cingulate, the lateral parietal lobes, and the medial and lateral temporal regions, and has been proposed to be involved in self-referential functions.17 Some DMN regions (posterior cingulate and precuneus) are highly connected with the rest of the brain, and recent studies have better elucidated the roles of DMN core regions as key components of specific subnetworks and functions. In a recent review, Leech et al. presented evidence that the posterior cingulate serves as a major hub involved in monitoring of the arousal state, and is particularly active in people who are prone to daydreaming, or during the retrieval of autobiographical memories.22 Utevsky et al., using a variety of cognitive tasks, observed increased connectivity between the precuneus and the frontoparietal network during task conditions, suggesting that the precuneus interacts with multiple brain networks depending on the cognitive status.23
Images represent group maps of 30 healthy young adults (20–40 years old) obtained using a data-driven approach. Networks are shown superimposed on the MNI152 standard space template image. All spatial maps are shown in radiological convention and are thresholded at z = 3. Red to yellow colours represent z-scores >3.
Other task-free networks that are commonly investigated in neurodegenerative diseases show good correspondence with the functional networks activated during the performance of a task. The salience network, comprising the dorsal anterior cingulate, bilateral orbitofrontal and insular cortices, guides behaviour by integrating limbic and autonomic information and relating it to social rules.24 The frontoparietal network, commonly segregated into left and right components, includes the dorsolateral prefrontal cortex, lateral parietal cortex and contralateral cerebellum, and subserves multiple cognitive paradigms, including memory, language and perception.24,25 This network can be further partitioned into a subnetwork, including prefrontal, temporal and parietal areas, that is associated with the language function. The executive network, which includes bilateral dorsolateral and medial prefrontal cortices, is associated with cognition, action inhibition and emotion.25 The sensorimotor network is anchored to the motor cortex (bilateral precentral and postcentral gyri, and supplementary motor area), and guides motor planning, execution and control.25 The visual network, which is commonly separated into medial and lateral portions of the occipital pole, is involved in visuoperceptual and visual control processing.25 A less well-characterized task-free network, potentially relevant to PD, is the cerebellothalamocortical network. This network includes the basal ganglia, the motor and premotor cortex and the cerebellum, and has a role in motor control.26
The correspondence between task-free and task-activated networks suggests that functional networks used by the brain when performing a task are continuously and dynamically active even when an individual is at rest and, therefore, reflect properties of functional brain organization.
Structural connectivity
Structural brain connectivity is generally assessed by means of diffusion tensor imaging (DTI). This technique measures the net diffusion of water molecules in brain tissues, revealing details about microstructural organization of white matter tissue. DTI can delineate white matter fibre orientation and provide indirect measures of tissue integrity. The two most widely used metrics of structural connectivity in neurodegenerative disorders are fractional anisotropy (FA) and mean diffusivity. These indices measure the anisotropy of the diffusion process and the magnitude of the diffusion, respectively. In the presence of damage to white matter fibres, such as axonal loss, Wallerian degeneration and demyelination, molecule diffusion is less hindered and FA is reduced. Tissue loss (including demyelination) can reduce local barriers to the movement of molecules in brain tissue, causing an increase in mean diffusivity.27 The main brain white matter tracts extracted with DTI are the cingulum, the fornix, the corpus callosum, the corticospinal tract (CST), the uncinate, the superior longitudinal fasciculus, and the inferior and fronto-occipital fasciculi.28 Recent advances in structural connectivity measurements, like high angular resolution diffusion imaging and diffusion kurtosis imaging, may provide additional markers of structural connectivity with more-detailed information about white matter microstructure.
Connectivity correlates of disease
Connectivity correlates of clinical phenotypes
Table 1 summarizes the available evidence that neurodegenerative diseases affect distinct functional and structural connectivity networks. Overall, evidence indicates that functional network abnormalities are associated with the topography and clinical severity of AD, behavioural variant FTD (bvFTD) and PSP, but in ALS, PD and HD, no clear association has yet emerged. Structural connectivity abnormalities correlate with the clinical phenotypes and disease severity in all major neurodegenerative diseases, but are topographically less specific.
Alzheimer disease
Task-free fMRI studies have identified consistent involvement of the DMN in AD.29,30,31,32,33,34,35 This network shows good topographic overlap with areas affected by pathology and neurodegeneration, that is, the posterior cingulate and preuneus, parietal cortex, hippocampus, and medial prefrontal cortex.5 Reduced connectivity (lower synchrony in the spontaneous BOLD fluctuations) in the DMN correlates with disease severity, as measured by the Clinical Dementia Rating (CDR) scale29,32,35 and cognitive–behavioural scores.31 There is consistent evidence that DMN damage tracks disease progression, as measured by cross-sectional studies at different disease stages,29,31,32,33 or longitudinal studies (Table 1).36
Structural connectivity changes also show good agreement with DMN topography in AD patients: significantly reduced FA and increased mean diffusivity values have been observed in limbic white matter tracts connecting the medial temporal lobe with the posterior cingulate.37,38,39,40 Moreover, damage to these regions has been shown to correlate with symptom severity and disease progression.37,38,39,40 White matter damage in AD is not confined to the DMN, however, and typically extends to frontal regions.37,38,39,40,41
Whether other networks are affected in AD is less clear. Considerable heterogeneity exists in terms of the networks investigated and findings across studies. It was initially suggested that the connectivity within the salience network is increased in AD.35 However, to date, this finding has not been replicated; indeed, two studies found reduced rather than increased salience network connectivity,32,42 and another showed no difference.30 The only network showing consistently enhanced connectivity in AD is the executive network.30,34,42 Several studies assessed the frontoparietal network in AD, but the findings were conflicting, variously showing reduced connectivity,32 mixed patterns30,42 or increased connectivity.34 Notwithstanding this uncertainty, an emerging feature seems to be the disruption of internetwork connectivity,32,43 possibly underlying functional disconnection and spread of pathology across interlinked networks.
Behavioural variant frontotemporal dementia
The salience network is consistently involved in bvFTD.35,42,44 As in AD, good topographical overlap is observed between this network and areas of grey matter atrophy, namely, the anterior cingulate and frontolimbic cortex.5 Reduced salience network connectivity has been shown to correlate with disease severity, as measured on the CDR scale35 and via behavioural scores.44 No study has yet investigated whether connectivity in the salience network tracks disease progression (Table 1).
Structural changes in bvFTD are congruent with salience network topography: frontal white matter tracts such as the uncinate fasciculus and the anterior cingulate show massive FA reduction, and damage to these tracts correlates with disease severity.41,45,46,47,48 As in AD, white matter damage extends beyond the salience network and involves posterior white matter tracts.41,45,46,47,48
Increased DMN connectivity in bvFTD has been reported by two studies,35,44 but two other studies found either reduced connectivity42 or mixed changes (both increased and reduced connectivity).49 Internetwork connectivity also seems to be disrupted in bvFTD.42
Progressive supranuclear palsy
In PSP, preliminary evidence from two studies supports the hypothesis of reduced cerebellothalamocortical network connectivity,50,51 in line with the topography of neurodegeneration in PSP.52 Damage to this network is associated with dementia severity,50 motor symptoms51 and cognitive impairment;51 however, further studies are required to confirm these associations. Multiple DTI studies have shown severe structural damage in PSP, which is not restricted to the cerebellothalamocortical network but involves all white matter tracts.51,53,54
There is conflicting evidence that reduced FA correlates with clinical symptoms in patients with PSP.51,54 A recent study suggests that whole-brain FA may be useful for the differential diagnosis of PSP, as it is very sensitive to white matter damage at the individual level.55
Amyotrophic lateral sclerosis
In ALS, the sensorimotor network has been investigated with task-free fMRI. Two studies found reduced connectivity in this network,56,57 while another reported a mixed pattern of both increased and reduced connectivity.58,59 Findings in ALS populations have been more consistent in terms of structural connectivity disruption. There is extensive evidence of reduced FA in the CST and corresponding motor network,60,61,62,63,64,65,66,67,68 and of an association between sensorimotor network damage and disease severity.63,66,67,68 Consistent with these data are the findings from two studies investigating ALS patients carrying superoxide dismutase 1 mutations, a dominantly inherited form of ALS associated with a less-severe clinical course and longer survival. Both studies showed relatively preserved CST connectivity in mutation carriers compared with sporadic cases.69,70 DTI changes in nonmotor tracts were reported by a recent study, and have been related to changes in social and emotional processing.71 Motor network damage has been suggested as a potential marker to track disease progression in ALS, with progressive expansion of white matter damage from the CST to extramotor areas.72,73,74 One recent longitudinal study examining sensorimotor network topology in ALS reported an expanding network of affected connectivity centralized to a motor disease epicentre.75
Four studies have combined functional and structural connectivity to assess sensorimotor disruption more comprehensively,60,61,76,77 and they all reported reduced structural connectivity. One study reported structural network deterioration with overall preserved functional connectivity.60 Two subsequent studies reported increased sensorimotor network connectivity.61,76 These studies also questioned whether increased connectivity reflected pathogenic or compensatory processes, and they reached opposite conclusions.61,76 Specifically, Agosta et al. showed that higher connectivity was associated with lower disease severity, suggesting compensatory mechanisms,76 whereas Douaud et al. found that increased connectivity was associated with a higher rate of disease progression, suggesting a pathogenic mechanism.61 A more recent study, examining the direct overlap between structural and functional connectivity changes, reported coherent loss of anatomical and functional interactions in the motor system in patients with ALS.77 No functional study has yet assessed whether functional sensorimotor network changes are sensitive to disease progression. Other networks investigated include the DMN and frontoparietal network, and reports mostly indicate increased connectivity in the DMN.56,57,78
Parkinson disease
Multiple task-free studies have investigated connectivity of the thalamocortical network in patients with PD, but the results have been mixed (Table 1). However, when patients were distinguished on the basis of their medication state (drug-naive PD, PD off levodopa, PD on levodopa), a more coherent picture emerged. Two studies scanned drug-naive PD patients and showed that thalamocortical connectivity was reduced in patients compared with controls,79,80 and administration of levodopa increased connectivity.79 A different pattern was observed in treated PD patients who were scanned in the 'off' state: studies found either increased connectivity81,82 or mixed patterns of both increased and reduced connectivity compared with controls.83,84,85 As in drug-naive PD, administration of levodopa normalized thalamocortical connectivity.81,83 Overall, these findings do not identify a connectivity signature for PD but suggest that acute levodopa administration restores normal thalamocortical connectivity. Finally, two studies assessed patients with PD under stable levodopa in the 'on' state and reported conflicting results, that is, either reduced86 or unchanged87 thalamocortical connectivity. Only two studies reported significant associations between connectivity abnormalities and disease severity (as assessed with the Unified PD Rating Scale);83,86 the majority failed to find such an association.81,82,84
Structural connectivity studies in patients with PD produced more-consistent results, indicating that white matter damage is initially confined to the brainstem and thalamus and subsequently spreads predominantly to frontal white matter tracts, paralleling the clinical88 and cognitive89 decline. Extramotor networks have been assessed by two studies, which showed reduced connectivity in the DMN90 and visual network.87 Two studies have revealed DTI changes in nonmotor tracts,88,89 and one of these studies related these changes to cognition.89 No longitudinal studies have been conducted to assess whether thalamocortical connectivity is sensitive to disease progression in PD.
Huntington disease
In HD, several resting-state networks have been investigated;91,92,93,94 however, a clear hypothesis on the possible 'target' networks is still lacking. Given the strong motor component in this disease, sensorimotor and basal ganglia–thalamocortical networks might be preferentially affected, although studies assessing these networks have provided mixed findings, some reporting reduced connectivity91,92 and others reporting increased connectivity or mixed patterns.93,94 These observations are not easy to interpret in view of the clear-cut and selective localization of HD pathology in the caudate nucleus, and the established role of this nucleus in large and well-defined corticostriatal loops (oculomotor, dorsolateral and ventral–orbital circuits).95
Structural connectivity studies in HD have been more consistent. FA reductions that correlate with disease severity have been reported in the basal ganglia and motor pathways.96,97,98 Nonmotor tracts have also been found to be affected in HD, and these changes seem to relate to cognition.96,97,99,100
Connectivity correlates of clinical subtypes
Table 2 summarizes the evidence that functional and structural connectivity biomarkers are associated with specific clinical subtypes within each neurodegenerative disease.
Alzheimer disease variants
In AD, the typical clinical phenotype is represented by late-onset amnestic AD. A proportion of patients, however, present with atypical phenotypes, for example, early-onset AD, or focal nonamnestic presentations such as posterior cortical atrophy or the logopenic variant of primary progressive aphasia (PPA). Preliminary evidence indicates that AD subtypes are associated with disruption of specific functional and structural networks. Two studies independently reported that early-onset AD is associated with damage to frontal networks—that is, the frontoparietal and salience networks101,102—in line with the neocortical phenotype associated with these forms. The involvement of frontal networks might also explain why these forms are difficult to distinguish from FTD in early disease stages. One study assessing structural connectivity in early-onset AD, however, did not find specific frontal involvement.103 Posterior cortical atrophy has been consistently associated with reduced connectivity to the visual network,101,104,105 and logopenic PPA with language network disruption.101,106,107,108 One study investigating functional networks in late-onset AD identified the anterior temporal network as being specifically involved in this form,102 although further confirmation is required.
Overall, these data indicate good topographical correspondence between affected networks and symptoms for each variant. Interestingly, the DMN is significantly affected in all variants101,102 and could, therefore, aid discrimination between AD and non-AD dementias in prodromal stages. An interesting question arising from these findings is whether the DMN represent an initial or final target of the disease, that is, whether pathology initially targets variant-specific networks and subsequently converges to the DMN, or vice versa.101 The answer to this question has important consequences for a better understanding of disease pathophysiology, and for biomarker development.
Frontotemporal dementia variants
FTD variants include behavioural (bvFTD) and language (semantic and nonfluent PPA) subtypes. Evidence indicates that each subtype is associated with distinct networks. As shown in the previous section, bvFTD is consistently associated with reduced connectivity of the salience network and frontolimbic white matter tracts.35,41,42,44,45,46,47,48 DTI studies have provided consistent evidence for reduced structural connectivity in frontotemporal pathways in semantic PPA, and fronto—parietotemporal pathways in nonfluent PPA.106,107,108 Evidence from task-free fMRI for the involvement of the language network in PPA variants is lacking,44 but it is reasonable to predict that this network will be markedly affected in all PPA variants. Finally, the uncinate fasciculus shows significant involvement across all FTD variants,41,103,104,105,106 suggesting that this biomarker may be central to understanding disease propagation across networks and variants.
Amyotrophic lateral sclerosis variants
For the main variants of ALS, data are still preliminary. The classic phenotype is characterized by upper and lower motor neuron signs, although a proportion of patients present, in addition, with behavioural and cognitive deficits compatible with a diagnosis of FTD (ALS–FTD). No task-free fMRI study has yet investigated whether these forms are associated with disruption of distinct functional networks.
Two recent anatomical studies assessed white matter abnormalities in these subtypes. One reported that reduced FA in the CST is specific to classic ALS, whereas reduced FA in the frontotemporal white matter tends to be more specific to ALS–FTD.109 While these findings are in line with clinical phenotypes, they need further replication, as a second study found no FA differences between subgroups.110
Progressive supranuclear palsy variants
In PSP, the most common presentation is Richardson syndrome (PSP-RS), characterized by early postural instability and falls, and supranuclear vertical gaze palsy. A smaller proportion of cases present with parkinsonian signs and generally show a less aggressive disease course (PSP-P). Only one DTI study has compared structural connectivity between PSP variants, reporting that infratentorial fibres and thalamic radiations are severely affected in PSP-RS and relatively spared in PSP-P,111 in line with disease topography and aggressiveness.
Connectivity correlates of molecular pathology
Whereas clinical phenotypes are 'downstream' events in disease pathophysiology, molecular pathology is 'upstream' (Figure 1). The pathological process starts many years prior to clinical onset,2 and identification of 'upstream' markers is critical for the development of effective interventions. Currently, the nature of the association between connectivity markers and molecular pathology in preclinical stages is largely unknown. This section reviews evidence on the relationships between connectivity abnormalities and molecular pathology in AD and FTD. Other diseases (PSP, ALS, PD and HD) will not be addressed owing to a lack of literature.
Alzheimer disease
Molecular pathology in AD is characterized by the deposition of two toxic proteins, amyloid-β (Aβ) and hyperphosphorylated tau.1 Cognitively normal elderly individuals with abnormal Aβ or tau levels are at a heightened risk of developing AD, and represent the ideal group to investigate preclinical AD.112 A large body of literature supports the view that amyloid pathology in these individuals, as assessed with PET or cerebrospinal fluid analysis, is associated with reduced DMN connectivity (Table 3).113,114,115,116,117 Similarly, carriers of the ε4 allele of the apolipoprotein E gene (APOE), by far the strongest genetic risk factor for sporadic late-onset AD,118 show DMN abnormalities that mirror those observed in clinical AD.119,120 These individuals also showed increased connectivity in frontal regions of the DMN and salience network, a finding that was not observed in those with amyloidosis.119,120 DTI studies showed lower structural connectivity in AD regions in elderly carriers of the APOE ε4 allele.121,122,123 The APOE ε4 allele is also associated with cortical Aβ deposition, and in order to tease out the independent contribution of this allele, one study investigated DMN changes in elderly APOE ε4 carriers who were Aβ-negative.124 The results confirmed the presence of DMN abnormalities similar to those observed in AD, as well as increased frontal connectivity.
These data raise the question of whether connectivity abnormalities reflect neuropathological or neurodevelopmental changes. Recent studies assessing structural and functional connectivity at a younger age seem to partially support the latter hypothesis, as they demonstrate that the APOE ε4 allele modulates connectivity strength decades before any clinical expression of neurodegenerative and/or ageing processes emerges. One study assessed the DMN in young adults (20–35 years of age), and found increased connectivity in APOE ε4 carriers.125 The findings of this study were subsequently independently confirmed.126 Similarly, a DTI study showed higher structural connectivity in APOE ε4 carriers than in noncarriers,127 although this finding was not confirmed by an independent study.128 Finally, a recent study assessing structural connectivity in infants showed lower connectivity in regions typically affected by AD, and increased connectivity in frontal regions.129 Overall it is difficult to reconcile this complex pattern of developmental and neurodegenerative connectivity changes into a unified framework, although these findings raise the possibility that the APOE ε4 allele is associated with a specific endophenotype.
Frontotemporal dementia
The pathological process underlying FTD—that is, frontotemporal lobar degeneration (FTLD)—has been elucidated in the past decade with the identification of two abnormal proteins, TAR DNA-binding protein 43 (TDP-43) and fused in sarcoma, which account for about 50–60% of FTD cases. The remaining cases are characterized by the presence of hyperphosphorylated tau (FTLD-tau), with only a small percentage of cases remaining unclassified.
To date, only one study has investigated the connectivity correlates of molecular pathology in FTD (Table 3).130 This study assessed structural connectivity in FTD patients with tau or TDP-43 pathology, defined on the basis of autopsy or genetic confirmation (MAPT for FTLD-tau, GRN or C9orf72 for FTLD-TDP), and showed greater damage in the superior longitudinal fasciculus in FTLD-tau than in FTLD-TDP.130 FA values in the superior longitudinal fasciculus discriminated molecular subtypes with 96% sensitivity and 100% specificity.130 Because the study was carried out in symptomatic patients, these results could indicate either a phenotypic difference or more-severe pathology in FTLD-tau.
A subsequent study by the same group selected 21 single nucleotide polymorphisms (SNPs) previously implicated in FTLD and investigated the structural connectivity correlates of these SNPs.131 The study identified three susceptibility SNPs, two conferring risk of the FTLD-TDP subtype (rs646776 and rs5848) and one for FTLD-tau pathology (rs1768208). The study included patients with sporadic FTD, and demonstrated that the three SNPs were associated with reduced connectivity of the midbrain and a mixed pattern of superior longitudinal fasciculus connectivity (Table 3). These preliminary data suggest a modulation effect of these susceptibility SNPs on structural connectivity.
With regard to functional connectivity, only one study to date has investigated connectivity changes associated with susceptibility genes in preclinical stages.132 The study included asymptomatic GRN mutation carriers (that is, with FTLD-TDP pathology) and focused on TMEM106B, a susceptibility factor associated with younger age at onset. The study showed that individuals carrying a risk polymorphism in TMEM106B (T/T) had reduced salience network and frontoparietal connectivity, a pattern of abnormalities similar to that observed in full-blown dementia.
Enhanced functional connectivity
As shown in the previous sections and in Table 1, enhanced functional connectivity (that is, increased synchrony of spontaneous BOLD fluctuations) is a common finding in task-free fMRI studies across a range of neurodegenerative diseases. This phenomenon has frequently been observed in motor diseases (for example, in PD and ALS), and occasionally also in cognitive–behavioural diseases (AD and FTD). The mechanism underlying these changes, however, is still unclear. Some authors have claimed compensatory mechanisms when increased connectivity was found associated with lower symptom severity.30,44,58,76,78,102 Other authors have also endorsed the 'compensatory theory', but with little evidence.57,84,87 Importantly, some studies showed an inverse relationship; that is, higher connectivity correlated with more-severe clinical symptoms35,44 or faster disease progression,58,60,61,83 suggesting a maladaptive and/or pathogenic mechanism rather than a compensatory phenomenon. A maladaptive response might reflect an unsuccessful attempt to recruit preserved neuronal areas to compensate for pathology, as well as a disrupted excitatory–inhibitory balance of damaged networks due to impending pathological processes. Overall, these findings indicate that enhanced connectivity is not a useful biomarker of disease severity in symptomatic individuals.
To better clarify the potential of enhanced connectivity as a disease biomarker at preclinical disease stages, we reviewed evidence from presymptomatic individuals with autosomal dominant forms of neurodegenerative diseases. According to the available literature (Table 4), evidence of over-recruitment is generally found in areas affected at late stages of the disease. In presymptomatic AD, one study reported greater connectivity in the frontal areas of the DMN,133 whereas another found no evidence of enhanced connectivity in this network.134 In FTD, one study found increased salience network connectivity in presymptomatic GRN mutation carriers,135 but others reported either reduced salience network connectivity136 or no changes.137 Whitwell et al.49 found increased connectivity in the posterior DMN of MAPT mutation carriers, a pattern similar to that observed in symptomatic bvFTD.35 In PD, only one study has assessed connectivity in mutation carriers (SCA2 gene), and showed reduced connectivity in the basal ganglia–thalamocortical network but enhanced connectivity in other networks affected later by the disease.138 Two studies conducted in presymptomatic HD showed no areas of increased connectivity in the sensorimotor91 or basal ganglia139 networks. At presymptomatic stages, FTD, ALS and HD were associated with reduced structural connectivity in regions affected early in the disease process (Table 4).136,137,140,141,142,143,144,145,146 Interestingly, in individuals with presymptomatic AD, two studies reported paradoxically lower mean diffusivity in the precuneus and cingulum,141,142 a pattern opposite to that observed in symptomatic mutation carriers.
With regard to the possible underlying mechanisms, as studies in presymptomatic individuals cannot investigate the association with cognitive or clinical decline, it is not possible to clarify whether changes in connectivity represent a pathogenic or compensatory mechanism, or simply a distinct endophenotype. However, the relatively stable finding of enhanced connectivity in areas affected by pathology at late stages of the disease process, concurrently with reduced connectivity in areas affected early on, might indicate a pathogenic mechanism such as disrupted excitatory–inhibitory balance between networks. A maladaptive mechanism is an unlikely explanation because of the lack of cognitive and clinical deficits. Caution is needed when interpreting these findings, however, because they are not consistently reported by all studies.
Future perspectives
Filling gaps in the field
The reviewed literature identifies two major gaps in the study of brain connectivity that should be addressed by future investigations. First, the trajectory of connectivity changes over the disease course is largely unclear. Cross-sectional and longitudinal studies in symptomatic individuals suggest that pathology spreads along brain connections,5,75 leading to the hypothesis that neurodegenerative diseases might be 'network-opathies'.147 However, a rigorous demonstration of when, where and how the pathology deposits and spreads along brain connections is still lacking, and will be an important goal of future research. The best approach would be a serial study covering the entire disease spectrum from normal to preclinical and clinical stages. Clearly, this is not a trivial undertaking in terms of resources, costs, and preclinical disease identification. In some cases, the latter issue is facilitated by the availability of in vivo disease markers, for example, markers of amyloidosis in AD, or susceptibility factors such as the APOE ε4 allele. Two large-scale population initiatives assessing individuals at risk of AD—Anti-Amyloid Treatment in Asymptomatic AD and the Alzheimer's Prevention Initiative—have recently commenced, and could serve as a reference for other studies. Alternatively, autosomal dominant forms of neurodegenerative diseases could represent a valid approach: studying these rare populations would enable inclusion of presymptomatic individuals who will certainly develop the disease. The Dominantly Inherited Alzheimer Network148 and the Genetic Frontotemporal Dementia Initiative149 are two examples of such an approach.
Second, future studies should aim to integrate multiple imaging modalities to address the possible interplay between structural and functional connectivity changes.150 Functional and structural connectivity are strongly linked: structural connectivity can predict functional connectivity; however, functional connections are also observed between regions that lack direct anatomical connections, possibly reflecting multisynaptic communication.151 To date, only a few studies have combined task-free fMRI and DTI to investigate network connectivity in neurological disorders.60,61 More-sophisticated approaches to combine structural and functional connectivity and more-complex measures of structural connectivity have recently been proposed,152,153 but have yet to be tested in the major neurodegenerative diseases. Furthermore, recent advances in the application of in silico simulations to model the effects of disease spread and structural damage on brain function provide a promising approach to examine neurodegenerative diseases.6,154 These methods may enable the creation of future predictive models to assess progressive disease effects in neurodegenerative disorders.
New targets
Neuronal communication has high metabolic costs.155 Brain 'hubs' receive a disproportionately high number of connections, and have been suggested to form a central 'rich-club' or 'core' in the brain, creating a central anatomical infrastructure for neuronal communication and integration of information across distal regions and networks.153,156 Brain hubs include core nodes of all major task-free networks,153 and these areas are disproportionately costly in terms of brain wiring and metabolic demand.155,157,158 These features could make the hubs especially vulnerable to pathology, either as a primary effect of the disease or as a secondary effect during disease progression. Indeed, simulation of damage to these regions was demonstrated to be especially disruptive for whole-brain connectivity.155,156 This finding is in line with the hypothesis of selective neuronal vulnerability, which suggests that certain well-defined neuronal circuits are specifically targeted by neurodegenerative processes.159 Related to this topic, an important question to address in AD is whether the higher functional connectivity observed in AD-specific brain regions in young adult APOE ε4 carriers125 may predispose these individuals to AD pathology through a network disruption mechanism starting decades before disease onset. Hubs and rich-club formation have been hypothesized to be involved in psychiatric disorders such as schizophrenia,160 but whether they have a primary role in neurodegenerative disorders, and in AD in particular, has yet to be established.
Vital insights might come in the near future from the availability of MRI sequences directly assessing brain metabolic costs and/or vascular contribution, such as arterial spin labelling. This sequence was designed in the early 1990s to measure cerebral blood flow, and it provides a more direct and quantitative physiological measurement than fMRI, without the drawbacks of PET. Perfusion-related alterations have been observed across a variety of neurodegenerative disorders,161 and these changes may reflect, or be prodromal to, pathophysiological processes. To date, arterial spin labelling has been infrequently used in research due to issues such as the low signal-to-noise ratio and the lack of standard sequences. These limitations are likely to be overcome in the near future owing to technical and harmonization efforts that are currently underway.162
Technical considerations
Technical factors such as field strength and the analytical method might account for the lack of consistent functional connectivity markers in PD, HD and ALS. The BOLD contrast is known to be significantly higher at 3 T than at 1.5 T, so scanners at lower field strength might be less sensitive to connectivity changes. Also, analytical approaches such as seed-based analysis might be less sensitive and specific than ICA-based approaches for identifying functional networks. When these differences are taken into account, however, no systemic bias emerges across studies that could explain the inconsistencies described above (Supplementary Table 1 online). Indeed, findings in AD and bvFTD seemed to be consistent even when different approaches were used.
Alternatively, the conflicting findings might be attributable to the presence of motion that is not detected by standard post-processing controls. Motion is one of the most important factors affecting fMRI studies: researchers have noted that motion induces spurious functional relationships between cortical regions,163 potentially influencing functional connectivity differences in patient studies. This phenomenon seems especially true for long-distance functional connections. As patients with neurodegenerative disease are more prone to movement than are healthy controls, and some disorders such as PD and ALS involve movement disturbances, general caution is needed in interpreting these findings, and methods to correct for these potential artefacts are advised.163 Furthermore, local atrophy may affect the fMRI signal and, thus, the computation of connectivity. This is a well-established issue, and correction for the contribution of atrophy is generally recommended as part of the statistical analysis. Of note, these issues are less of a concern in studies on preclinical disease stages, since atrophy is minimal and patients tend to move less. Other possible explanations for the conflicting findings might be the small sample sizes, and functional connectivity variability related to the internal status of the patient.
Because the BOLD signal is an indirect measure of neuronal activity, and depends largely on vascular components (cerebral blood flow, cerebral blood volume and vascular compliance), brain oxygen metabolism and the coupling between these measures, any change in one of these factors may affect both the amplitude and the magnitude of the signal. While this is not a major problem when BOLD fMRI is used for anatomical localization of neuronal activity, quantitative interpretation of the signal in diseased populations is less straightforward. Baseline perfusion measures and a more comprehensive fMRI analysis model may be essential for inferring group differences.164
Finally, test–retest reliability is critical in view of future longitudinal studies. Several task-free networks discussed in this Review (DMN, sensorimotor, frontoparietal and visual) and the major white matter tracts show good reliability over 12 months.165,166 However, reliability figures vary largely across networks and pathways, and future studies will need to demonstrate sufficient reliability for clinical use.
Conclusions
A summary of the reviewed literature is provided in Figure 3. Overall, current evidence supports the validity of task-free fMRI functional connectivity as a marker of clinical phenotype and disease severity in AD and bvFTD. However, evidence is still weak on its validity in ALS, PD and HD. In these latter neurodegenerative diseases, which are characterized by motor phenotypes, structural connectivity markers are stronger candidate biomarkers of clinical phenotype and disease severity. Figure 4 illustrates the correspondence between structural and functional connectivity findings in some of the neurodegenerative diseases discussed in this Review.
Functional connectivity signatures are shown in solid grey squares, and structural connectivity signatures are shown in orange squares. Brain signatures of molecular pathology are the subject of current research and are shown in dotted arrows. Abbreviations: ALS, amyotrophic lateral sclerosis; bvFTD, behavioural variant frontotemporal dementia; DLB, dementia with Lewy bodies; DMN, default mode network; EOAD, early-onset Alzheimer disease; LOAD, late-onset Alzheimer disease; PCA, posterior cortical atrophy; PD, Parkinson disease; PPA, primary progressive aphasia; PSP, progressive supranuclear palsy; SMN, sensorimotor network.
For each disease, the panel shows the predicted pattern of functional and structural connectivity disruption. The predicted pattern is defined on the basis of the pathophysiology of the disease. Green corresponds to functional networks and structural pathways (light green and dark green, respectively) that fulfil the prediction, whereas red corresponds to networks and pathways that do not fulfil the prediction, according to the reviewed evidence.
From a translational perspective, several factors, which could limit the clinical use of connectivity markers, should be mentioned. First, the current literature is largely based on group-level comparisons, and the specificity and sensitivity of these biomarkers must be established. A few studies carried out in patients with AD and bvFTD showed that the added value of these markers might be only incremental compared with standard markers of neurodegeneration.40,167 Future studies in patients at preclinical stages will indicate whether connectivity markers can outperform structural MRI markers early in the disease course. Second, functional connectivity findings are inconsistent in motor phenotypes. As mentioned above, structural connectivity markers might be more useful for these diseases.
Finally, owing to the increasing availability of in vivo markers of pathology, such as cerebrospinal fluid-derived or PET-derived amyloid and tau signatures, the molecular correlates of abnormal connectivity can now be studied. As shown in the section on connectivity correlates of molecular pathology in AD, findings in this area are very promising. The molecular correlates of connectivity abnormalities in the FTD spectrum are largely unexplored, but future studies in this field will enable identification of the determinants of the spread of pathology across distinct neuronal networks, and possibly shed light on the mechanisms underlying phenotypic variability.
Review criteria
Articles were identified by searching PubMed for the following terms: “brain connectivity”, “structural connectivity”, “functional connectivity”, “resting-state functional MRI”, “diffusion tensor imaging”, “dementia”, “neurodegenerative disorders”, “Alzheimer”, “frontotemporal dementia”, “Parkinson”, “amyotrophic lateral sclerosis”, “progressive supranuclear palsy”, and “Huntington”. Articles were also identified through searches of the authors' own files. Reference lists of the identified papers were examined for further leads. The search was limited to full-text manuscripts published in English between 2004 and 2014 (except for theoretical or methodological papers). The final selection was based on originality and relevance, as judged by the authors.
References
Taylor, J. P., Hardy, J. & Fischbeck, K. H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002).
Villemagne, V. L. et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 12, 357–367 (2013).
Frisoni, G. B., Fox, N. C., Jack, C. R. Jr, Scheltens, P. & Thompson, P. M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 6, 67–77 (2010).
Whitwell, J. L. & Josephs, K. A. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat. Rev. Neurol. 8, 131–142 (2012).
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L. & Greicius, M. D. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52 (2009).
Raj, A., Kuceyeski, A. & Weiner, M. A network diffusion model of disease progression in dementia. Neuron 73, 1204–1215 (2012).
Moeller, J. R., Strother, S. C., Sidtism, J. J. & Rottenberg, D. A. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J. Cereb. Blood Flow Metab. 7, 649–658 (1987).
Horwitz, B. Simulating functional interactions in the brain: a model for examining correlations between regonal cerebral metabolic rates. Int. J. Biomed. Comput. 26, 149–170 (1990).
Friston, K. J. Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 2, 56–78 (1994).
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Greicius, M. D. et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437 (2007).
Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101, 4637–4642 (2004).
Sorg, C. et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 104, 18760–18765 (2007).
Damoiseaux, J. S. et al. Consistent resting-state networks across healthy subjects. Proc. Natl Acad. Sci. USA. 103, 13848–13853 (2006).
Chen, S. et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 1239, 141–151 (2008).
Goldman, R. I., Stern, J. M., Engel, J. Jr & Cohen, M. S. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13, 2487–2492 (2002).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Biswal, B. B. et al. Toward discovery science of human brain function. Proc. Natl Acad. Sci. USA 107, 4734–4739 (2010).
Fukunaga, M. et al. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J. Cereb. Blood Flow Metab. 28, 1377–1387 (2008).
Vincent, J. L. et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 (2007).
Lu, H. et al. Rat brains also have a default mode network. Proc. Natl Acad. Sci. USA 109, 3979–3984 (2012).
Leech, R. & Sharp, D. J. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32 (2014).
Utevsky, A. V., Smith, D. V. & Huettel, S. A. Precuneus is a functional core of the default-mode network. J. Neurosci. 34, 932–940 (2014).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Smith, S. M. et al. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13040–13045 (2009).
Zhang, D. et al. Intrinsic functional relations between human cerebral cortex and thalamus. J. Neurophysiol. 100, 1740–1748 (2008).
Alexander, A. L., Lee, J. E., Lazar, M. & Field, A. S. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329 (2007).
Catani, M. & Thiebaut de Schotten, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132 (2008).
Petrella, J. R., Sheldon, F. C., Prince, S. E., Calhoun, V. D. & Doraiswamy, P. M. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 76, 511–517 (2011).
Agosta, F. et al. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging 33, 1564–1578 (2012).
Binnewijzend, M. A. et al. Resting-state fMRI changes in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging 33, 2018–2028 (2012).
Brier, M. R. et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 32, 8890–8899 (2012).
Zhang, H. Y. et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology 256, 598–606 (2010).
Zamboni, G. et al. Resting functional connectivity reveals residual functional activity in Alzheimer's disease. Biol. Psychiatry 74, 375–383 (2013).
Zhou, J. et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133, 1352–1367 (2010).
Damoiseaux, J. S., Prater, K. E., Miller, B. L. & Greicius, M. D. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol. Aging 33, 828.e19–828.e30 (2012).
Pievani, M. et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Hum. Brain Mapp. 31, 1862–1875 (2010).
Huang, H. et al. Distinctive disruption patterns of white matter tracts in Alzheimer's disease with full diffusion tensor characterization. Neurobiol. Aging 33, 2029–2045 (2012).
Acosta-Cabronero, J., Alley, S., Williams, G. B., Pengas, G. & Nestor, P. J. Diffusion tensor metrics as biomarkers in Alzheimer's disease. PLoS ONE 7, e49072 (2012).
Douaud, G. et al. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. J. Neurosci. 33, 2147–2155 (2013).
Zhang, Y. et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain 132, 2579–2592 (2009).
Filippi, M. et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex 49, 2389–2401 (2013).
Song, J. et al. Aberrant functional organization within and between resting-state networks in AD. PLoS ONE 8, e63727 (2013).
Farb, N. A. et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex 49, 1856–1873 (2013).
Borroni, B. et al. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch. Neurol. 64, 246–251 (2007).
Hornberger, M., Geng, J. & Hodges, J. R. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain 134, 2502–2512 (2011).
Mahoney, C. J. et al. Profiles of white matter tract pathology in frontotemporal dementia. Hum. Brain Mapp. 35, 4163–4179 (2014).
Tovar-Moll, F. et al. White matter tract damage in the behavioral variant of frontotemporal and corticobasal dementia syndromes. PLoS ONE 9, e102656 (2014).
Whitwell, J. L. et al. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology 77, 866–874 (2011).
Gardner, R. C. et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann. Neurol. 73, 603–616 (2013).
Whitwell, J. L. et al. Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat. Disord. 17, 599–605 (2011).
Boxer, A. L. et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 63, 81–86 (2006).
Canu, E. et al. Diffusion tensor magnetic resonance imaging tractography in progressive supranuclear palsy. Mov. Disord. 26, 1752–1755 (2011).
Whitwell, J. L. et al. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch. Neurol. 68, 753–760 (2011).
Sajjadi, S. A. et al. Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain 136, 2253–2261 (2013).
Mohammadi, B. et al. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp. Neurol. 217, 147–153 (2009).
Tedeschi, G. et al. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol. Aging 33, 886–898 (2012).
Zhou, F. et al. Altered motor network functional connectivity in amyotrophic lateral sclerosis: a resting-state functional magnetic resonance imaging study. Neuroreport 24, 657–662 (2013).
Zhou, F. et al. Alterations in regional functional coherence within the sensory-motor network in amyotrophic lateral sclerosis. Neurosci. Lett. 558, 192–196 (2014).
Verstraete, E. et al. Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS ONE 5, e13664 (2010).
Douaud, G., Filippini, N., Knight, S., Talbot, K. & Turner, M. R. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain 134, 3470–3479 (2011).
Verstraete, E., Veldink, J. H., Mandl, R. C., van den Berg, L. H. & van den Heuvel, M. P. Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS ONE 6, e24239 (2011).
Agosta, F. et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am. J. Neuroradiol. 31, 1457–1461 (2010).
Rose, S. et al. Direct evidence of intra- and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: an automated MRI structural connectivity study. Neuroimage 59, 2661–2669 (2012).
Bede, P. et al. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81, 361–369 (2013).
Trojsi, F. et al. Motor and extramotor neurodegeneration in amyotrophic lateral sclerosis: a 3 T high angular resolution diffusion imaging (HARDI) study. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 553–561 (2013).
Thivard, L. et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J. Neurol. Neurosurg. Psychiatry 78, 889–892 (2007).
Zhang, J. et al. Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J. Neurol. 261, 412–421 (2014).
Stanton, B. R. et al. Diffusion tensor imaging in sporadic and familial (D90A SOD1) forms of amyotrophic lateral sclerosis. Arch. Neurol. 66, 109–115 (2009).
Blain, C. R. et al. Differential corticospinal tract degeneration in homozygous 'D90A' SOD-1 ALS and sporadic ALS. J. Neurol. Neurosurg. Psychiatry 82, 843–849 (2011).
Crespi, C. et al. Microstructural white matter correlates of emotion recognition impairment in amyotrophic lateral sclerosis. Cortex 53, 1–8 (2014).
Sage, C. A., Peeters, R. R., Görner, A., Robberecht, W. & Sunaert, S. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34, 486–499 (2007).
Keil, C. et al. Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci. 13, 141 (2012).
Senda, J. et al. Progressive and widespread brain damage in ALS: MRI voxel-based morphometry and diffusion tensor imaging study. Amyotroph. Lateral Scler. 12, 59–69 (2011).
Verstraete, E., Veldink, J. H., van den Berg, L. H. & van den Heuvel, M. P. Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Hum. Brain Mapp. 35, 1351–1361 (2014).
Agosta, F. et al. Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb. Cortex 21, 2291–2298 (2011).
Schmidt, R. et al. Correlation between structural and functional connectivity impairment in amyotrophic lateral sclerosis. Hum. Brain Mapp. 35, 4386–4395 (2014).
Agosta, F. et al. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol. Aging 34, 419–427 (2013).
Esposito, F. et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain 136, 710–725 (2013).
Luo, C. et al. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol. Aging 35, 431–441 (2014).
Kwak, Y. et al. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front. Syst. Neurosci. 4, 143 (2010).
Yu, R., Liu, B., Wang, L., Chen, J. & Liu, X. Enhanced functional connectivity between putamen and supplementary motor area in Parkinson's disease patients. PLoS ONE 8, e59717 (2013).
Wu, T. et al. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci. Lett. 460, 6–10 (2009).
Helmich, R. C. et al. I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb. Cortex 20, 1175–1186 (2010).
Choe, I. H., Yeo, S., Chung, K. C., Kim, S. H. & Lim, S. Decreased and increased cerebral regional homogeneity in early Parkinson's disease. Brain Res. 1527, 230–237 (2013).
Hacker, C. D., Perlmutter, J. S., Criswell, S. R., Ances, B. M. & Snyder, A. Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain 135, 3699–3711 (2012).
Göttlich, M. et al. Altered resting state brain networks in Parkinson's disease. PLoS ONE 8, e77336 (2013).
Agosta, F. et al. The topography of brain damage at different stages of Parkinson's disease. Hum. Brain Mapp. 34, 2798–2807 (2013).
Melzer, T. R. et al. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 80, 1841–1849 (2013).
Tessitore, A. et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79, 2226–2232 (2012).
Dumas, E. M. et al. Reduced functional brain connectivity prior to and after disease onset in Huntington's disease. Neuroimage Clin. 2, 377–384 (2013).
Poudel, G. R. et al. Abnormal synchrony of resting state networks in premanifest and symptomatic Huntington disease: the IMAGE-HD study. J. Psychiatry Neurosci. 39, 87–96 (2014).
Werner, C. J. et al. Altered resting-state connectivity in Huntington's disease. Hum. Brain Mapp. 35, 2582–2593 (2014).
Quarantelli, M. et al. Default-mode network changes in Huntington's disease: an integrated MRI study of functional connectivity and morphometry. PLoS ONE 8, e72159 (2013).
Grahn, J. A., Parkinson, J. A. & Owen, A. M. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav. Brain Res. 199, 53–60 (2009).
Novak, M. J. et al. White matter integrity in premanifest and early Huntington's disease is related to caudate loss and disease progression. Cortex 52, 98–112 (2014).
Della Nave, R. et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR Am. J. Neuroradiol. 31, 1675–1681 (2010).
Bohanna, I., Georgiou-Karistianis, N. & Egan, G. F. Connectivity-based segmentation of the striatum in Huntington's disease: vulnerability of motor pathways. Neurobiol. Dis. 42, 475–481 (2011).
Rosas, H. D. et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical “disconnection”. Neuroimage 49, 2995–3004 (2010).
Bohanna, I. et al. Diffusion tensor imaging in Huntington's disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 5, 171–180 (2011).
Lehmann, M. et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer's disease. Proc. Natl Acad. Sci. USA 110, 11606–11611 (2013).
Gour, N. et al. Functional connectivity changes differ in early and late-onset Alzheimer's disease. Hum. Brain Mapp. 35, 2978–2994 (2014).
Canu, E. et al. White matter microstructural damage in Alzheimer's disease at different ages of onset. Neurobiol. Aging 34, 2331–2340 (2013).
Migliaccio, R. et al. Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex 48, 1298–1309 (2012).
Duning, T. et al. Pattern and progression of white-matter changes in a case of posterior cortical atrophy using diffusion tensor imaging. J. Neurol. Neurosurg. Psychiatry 80, 432–436 (2009).
Agosta, F. et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb. Cortex 22, 2705–2714 (2012).
Zhang, Y. et al. MRI signatures of brain macrostructural atrophy and microstructural degradation in frontotemporal lobar degeneration subtypes. J. Alzheimers Dis. 33, 431–444 (2013).
Whitwell, J. L. et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 74, 1279–1287 (2010).
Lillo, P. et al. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS ONE 7, e43993 (2012).
Rajagopalan, V., Yue, G. H. & Pioro, E. P. Brain white matter diffusion tensor metrics from clinical 1.5 T MRI distinguish between ALS phenotypes. J. Neurol. 260, 2532–2540 (2013).
Agosta, F. et al. Diffusion tensor MRI contributes to differentiate Richardson's syndrome from PSP-parkinsonism. Neurobiol. Aging 33, 2817–2826 (2012).
Vos, S. J. et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965 (2013).
Hedden, T. et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 29, 12686–12694 (2009).
Sheline, Y. I. et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 67, 584–587 (2010).
Drzezga, A. et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 134, 1635–1646 (2011).
Wang, L. et al. Cerebrospinal fluid Aβ42, phosphorylated Tau181, and resting-state functional connectivity. JAMA Neurol. 70, 1242–1248 (2013).
Brier, M. R. et al. Functional connectivity and graph theory in preclinical Alzheimer's disease. Neurobiol. Aging 35, 757–768 (2014).
Strittmatter, W. J. et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 1977–1981 (1993).
Machulda, M. M. et al. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol. 68, 1131–1136 (2011).
Patel, K. T. et al. Default mode network activity and white matter integrity in healthy middle-aged ApoE4 carriers. Brain Imaging Behav. 7, 60–67 (2013).
Brown, J. A. et al. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc. Natl Acad. Sci. USA 108, 20760–20765 (2011).
Honea, R. A., Vidoni, E., Harsha, A. & Burns, J. M. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J. Alzheimers Dis. 18, 553–564 (2009).
Chiang, G. C., Zhan, W., Schuff, N. & Weiner, M. W. White matter alterations in cognitively normal apoE ε2 carriers: insight into Alzheimer resistance? AJNR Am. J. Neuroradiol. 33, 1392–1397 (2012).
Sheline, Y. I. et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 30, 17035–17040 (2010).
Filippini, N. et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl Acad. Sci. USA 106, 7209–7214 (2009).
Dennis, N. A. et al. Temporal lobe functional activity and connectivity in young adult APOE e4 carriers. Alzheimers Dement. 6, 303–311 (2010).
Dowell, N. G. et al. MRI of carriers of the apolipoprotein E e4 allele-evidence for structural differences in normal-appearing brain tissue in e4+ relative to e4− young adults. NMR Biomed. 26, 674–682 (2013).
O'Dwyer, L. et al. White matter differences between healthy young ApoE4 carriers and non-carriers identified with tractography and support vector machines. PLoS ONE 7, e36024 (2012).
Dean, D. C. et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 71, 11–22 (2014).
McMillan, C. T. et al. White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 84, 949–955 (2013).
McMillan, C. T. et al. Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiol. Aging 35, 1473–1482 (2014).
Premi, E. et al. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 71, 216–221 (2014).
Sala-Llonch, R. et al. Evolving brain functional abnormalities in PSEN1 mutation carriers: a resting and visual encoding fMRI study. J. Alzheimers Dis. 36, 165–175 (2013).
Chhatwal, J. P. et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81, 736–744 (2013).
Borroni, B. et al. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol. Aging 33, 2506–2520 (2012).
Dopper, E. G. et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology 80, 814–823 (2013).
Pievani, M. et al. Pattern of structural and functional brain abnormalities in asymptomatic granulin mutation carriers. Alzheimers Dement. http://dx.doi.org/10.1016/j.jalz.2013.09.009.
Wu, T. et al. Preclinical and clinical neural network changes in SCA2 parkinsonism. Parkinsonism Relat. Disord. 19, 158–164 (2013).
Unschuld, P. G. et al. Impaired cortico-striatal functional connectivity in prodromal Huntington's disease. Neurosci. Lett. 514, 204–209 (2012).
Ringman, J. M. et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain 130, 1767–1776 (2007).
Ryan, N. S. et al. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain 136, 1399–1414 (2013).
Fortea, J. et al. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J. Alzheimers Dis. 22, 909–922 (2010).
Borroni, B. et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res. 11, 585–595 (2008).
Ng, M. C. et al. Abnormal diffusion tensor in nonsymptomatic familial amyotrophic lateral sclerosis with a causative superoxide dismutase 1 mutation. J. Magn. Reson. Imaging 27, 8–13 (2008).
Dumas, E. M. et al. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington's disease. Hum. Brain Mapp. 33, 203–212 (2012).
Rosas, H. D. et al. Diffusion tensor imaging in presymptomatic and early Huntington's disease: Selective white matter pathology and its relationship to clinical measures. Mov. Disord. 21, 1317–1325 (2006).
Warren, J. D., Rohrer, J. D. & Hardy, J. Disintegrating brain networks: from syndromes to molecular nexopathies. Neuron 73, 1060–1062 (2012).
Dian Observational Study. Dominantly Inherited Alzheimer Network [online], (2013).
GENFI: the Genetic Frontotemporal Dementia Initiative. UCL [online], (2014).
Damoiseaux, J. S. & Greicius, M. D. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 213, 525–533 (2009).
Honey, C. J. et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040 (2009).
Hagmann, P. et al. MR connectomics: principles and challenges. J. Neurosci. Methods 194, 34–45 (2010).
van den Heuvel, M. P. & Sporns, O. An anatomical substrate for integration among functional networks in human cortex. J. Neurosci. 33, 14489–14500 (2013).
Zhou, J., Gennatas, E. D., Kramer, J. H., Miller, B. L. & Seeley, W. W. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73, 1216–1227 (2012).
Bullmore, E. & Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349 (2012).
van den Heuvel, M. P. & Sporns, O. Rich-club organization of the human connectome. J. Neurosci. 31, 15775–15786 (2011).
van den Heuvel, M. P., Kahn, R. S., Goñi, J. & Sporns, O. High-cost, high-capacity backbone for global brain communication. Proc. Natl Acad. Sci. USA 109, 11372–11377 (2012).
Collin, G., Sporns, O., Mandl, R. C. & van den Heuvel, M. P. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb. Cortex 24, 2258–2267 (2014).
Morrison, J. H. & Hof, P. R. Life and death of neurons in the aging brain. Science 278, 412–419 (1997).
van den Heuvel, M. P. et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 70, 783–792 (2013).
Hu, W. T. et al. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 75, 881–888 (2010).
Arterial spin labelling Initiative in Dementia (AID). COST: European Cooperation in Science and Technology [online], (2014).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Uludag˘, K. et al. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage 23, 148–155 (2004).
Danielian, L. E., Iwata, N. K., Thomasson, D. M. & Floeter, M. K. Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. Neuroimage 49, 1572–1580 (2010).
Zuo, X. N. et al. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage 49, 2163–2177 (2010).
McMillan, C. T. et al. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology 78, 1761–1768 (2012).
Acknowledgements
N.F. is funded by the HDH Wills 1965 Charitable Trust. M.P.v.d.H. is supported by a Fellowship from the Brain Center Rudolf Magnus and a VENI grant from the Dutch Council for Research (NWO).
Author information
Authors and Affiliations
Contributions
M.P. and G.B.F. developed the architecture of the manuscript. M.P. wrote the initial draft, which was completed, edited and reviewed for important intellectual content by N.F., M.P.v.d.H., S.F.C. and G.B.F. M.P. and G.B.F. prepared Figure 1, Figure 3 and Figure 4, and N.F. prepared Figure 2. All the authors have seen and approved the final version.
Corresponding author
Ethics declarations
Competing interests
G.B.F. has served on the advisory boards for Lilly, BMS, Bayer, Lundbeck, Elan, AstraZeneca, Pfizer, Baxter, Taurx and Wyeth, and has received research support from Wyeth, Lilly and Lundbeck Italia. The other authors declare no competing interests.
Supplementary information
Supplementary Table 1
Technical variability (magnetic field strength and post-processing method) across studies on resting-state functional connectivity MRI (PDF 157 kb)
Rights and permissions
About this article
Cite this article
Pievani, M., Filippini, N., van den Heuvel, M. et al. Brain connectivity in neurodegenerative diseases—from phenotype to proteinopathy. Nat Rev Neurol 10, 620–633 (2014). https://doi.org/10.1038/nrneurol.2014.178
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.178
This article is cited by
-
Locating causal hubs of memory consolidation in spontaneous brain network in male mice
Nature Communications (2023)
-
Different MRI structural processing methods do not impact functional connectivity computation
Scientific Reports (2023)
-
Functional brain networks in the evaluation of patients with neurodegenerative disorders
Nature Reviews Neurology (2023)
-
The BrainLat project, a multimodal neuroimaging dataset of neurodegeneration from underrepresented backgrounds
Scientific Data (2023)
-
Connectomics-based resting-state functional network alterations predict suicidality in major depressive disorder
Translational Psychiatry (2023)