Abstract
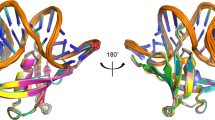
The archaeon Sulfolobus solfataricus expresses large amounts of a small basic protein, Sso7d, which was previously identified as a DNA-binding protein possibly involved in compaction of DNA. We have determined the solution structure of Sso7d. The protein consists of a triple-stranded anti-parallel β-sheet onto which an orthogonal double-stranded β-sheet is packed. This topology is very similar to that found in eukaryotic Src homology-3 (SH3) domains. Sso7d binds strongly (Kd < 10 μM) to double-stranded DNA and protects it from thermal denaturation. In addition, we note that ɛ-mono-methylation of lysine side chains of Sso7d is governed by cell growth temperatures, suggesting that methylation is related to the heat-shock response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kornberg, R. & Lorch, Y. Chromatin structure and transcription. A. Rev. Cell. Biol. 8, 563–587 (1992).
Schmid, M.B. More than just “histone-like” proteins. Cell 63, 451–453 (1990).
Dijk, J. & Reinhardt, R. The structure of DNA-binding proteins from eu and archaebacteria. In Bacterial chromatin (Eds, Gualerzi, C.O. and Pon, C.L.) 185–218, (Springer-Verlag, Berlin, 1986)
Pettijohn, D. Histone-like proteins and bacterial chromosome structure. J. biol. Chem. 236, 12793–12796 (1988).
Drlica, K. & Rouviere-Yaniv, J. Histonelike proteins in bacteria. Microbiol. Rev. 51, 301–319 (1987).
Grayling, R.A., Sandman, K. & Reeve, J.N. Archaeal DNA binding proteins and chromosome structure. System. appl. Micobiol. 16, 582–590 (1994).
Brock, T.D., Brock, K.M., Belly, R.T. & Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 84, 54–68 (1972).
Thomm, M., Stetter, K.O. & Zillig, W. Histone-like proteins in eu and archaebacteria. Zentralbl. Bakteriol. Mikrobiol. Hyg., I. Abt. Orig. C 3, 128–139 (1982).
Kimura, M., Kimura, J., Davie, P., Reinhardt, R. & Dijk, J. The amino acid sequence of a small DNA binding protein from the archaebacterium Sulfolobus solfataricus. FEBS Letts. 176, 176–178 (1984).
Grote, M., Dijk, J. & Reinhardt, R. Ribosomal and DNA binding proteins of the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biochim. biophys. Acta 873, 405–413 (1986).
Choli, T., Wittmann-Liebold, B. & Reinhardt, R. Microsequence analysis of DNA-binding proteins 7a, 7b and 7e from the archaebacterium Sulfolobus acidocaldarius. J. biol. Chem. 263, 7087–7093 (1988).
Choli, T., Henning, P., Wittmann-Liebold, B. & Reinhardt, R. Isolation, characterization and microsequence analysis of a small basic methylated DNA-binding protein from the archaebacterium Sulfolobus solfataricus. Biochim. biophys. Acta 950, 193–203 (1988).
Saraste, M., Sibbald, P.R. & Wittinghofer, A., The P-loop - a common motif in ATP- and GTP-binding proteins. Trends biochem. Sci 15, 430–434 (1990).
Guagliardi, A., Cerchia, L., Camardella, L., Rossi, M. & Bartolucci, S. DBF (disulfide bond forming) enzyme from the hyperthermophilic archaebacterium Sulfolobus solfataricus behaves like a molecular chaperone. Biocatalysis (in the press)
Fusi, P., Tedeschi, G., Aliverti, A., Ronchi, S., Tortora, P. & Guerritore, P. Ribonucleases from the extreme thermophilic archaebacterium S. solfataricus. Eur. J. Biochem. 211, 305–310 (1993).
Jentoft, N. & Dearborn, D. Protein labeling by reductive alkylation. Meths. Enzymol. 91, 570–579 (1983).
Stein, D.B. & Searcy, D.G. Physiologically important stabilization of DNA by a prokaryotic histone-like protein. Science 202, 219–221 (1978).
Wüthrich, K. NMR of proteins and nucleic acids. (Wiley, New York, 1986)
Bax, A. Two-dimensional NMR and protein structure. A. Rev. Biochem. 58, 223–256 (1989).
Pawson, T. & Schlessinger, J. SH2 and SH3 domains. Curr. Biol. 3, 434–442 (1993).
Kuriyan, J. & Cowburn, D. Structures of SH2 and SH3 domains. Curr. Opin. struct. Biol. 3, 828–837 (1993).
Koyama, S. et al. Structure of the P13K SH3 domain and analysis of the SH3 family. Cell 72, 945–952 (1993).
Musacchio, A., Noble, M., Pauptit, R., Wierenga, R. & Saraste, M. Crystal structure of a src-homology 3 (SH3) domain. Nature 359, 851–855 (1992).
Noble, M.E.M., Musacchio, A., Saraste, M., Courtneidge, S.A. & Wierenga, R.K. Crystal structure of the SH3 domain in human Fyn; comparison of the three-dimansional structures of SH3 domains in tyrosine kinases and spectrin. EMBO J. 12, 2617–2624 (1993).
Falzone, C.J., Kao, Y.-H., Zhao, J., Bryant, D.A. & Lecomte, J.T.J. Three-dimensional solution structure of PsaE from the cyanobacterium Synechococcus sp. strain PCC 7002, a photosystem I protein that shows structural homology with SH3 domains. Biochemistry 33, 6052–6062 (1994).
Wilson, K.P., Shewchuk, L.M., Brennan, R.G., Otsuka, J. & Matthews, B.W. Eschericia coli biotin holoenzyme synthetase/bio repressor crystal structure delineates the biotin- and DNA-binding domains. Proc. natn. Acad. Sci. U.S.A. 89, 9257–9261 (1992).
Zillig, W. et al. The sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerase. Arch. Microbiol. 125, 259–269 (1980).
Cantor, C.R., Schimmel, P. Biophysical chemistry. Part II, Chapter 7, (Freeman, San Francisco, 1980)
Lundbäck, T., Zilliacus, J., Gustafsson, J.-Å., Carlstedt-Duke, J. & Härd, T. Thermodynamics of sequence-specific glucocorticoid receptor-DNA interactions. Biochemistry 33, 5955–5965 (1994).
States, D.J., Haberkorn, R.A. & Ruben, R.J. A 2D NMR experiment with pure absorption phase in four quadrants. J. magn. Reson. 48, 286–295 (1982).
Rance, M. et al. Improved spectral resolution in COSY 1H NMR spectra of proteins via double quantum coherence. Biochem biophys. Res. Comm. 117, 479–485 (1983).
Macura, A. & Ernst, R.R. Elucidation of crossrelaxation in liquids by 2D NMR spectroscopy. Molec. Phys. 41, 95–117 (1980).
Griesinger, C., Otting, G., Wüthrich, K. & Ernst, R.R. Clean TOCSY for 1H spin system identification in macromolecules. J. Am. chem. Soc. 110, 7870–7872 (1988).
Davis, A.L., Keeler, J., Laue, E.D. & Moskau, D. Experiments for recording pure-absorption heteronuclear correlation spectra using pulsed field gradients. J. magn. Reson. 98, 207–216 (1992).
Smallcombe, S.H. Solvent suppression with symmetrically-shifted pulses. J. Am. chem. Soc. 115, 4776–4785 (1993).
Patt, S.L. Single- and multiple-frequency-shifted laminar pulses. J. magn Reson. 96, 94–102 (1992).
Brown, S.C., Weber, P.L. & Mueller, L. Toward complete 1H NMR spectra in proteins. J. magn. Reson. 77, 166–169 (1988).
Kraulis, P.J. ANSIG: a program for the assignment of protein 1H 2D NMR spectra by interactive computer graphics. J. magn. Reson. 84, 627–633 (1989).
Hyberts, S., Märki, W. & Wagner, G. Stereospecific assignments of side-chain protons and characterization of torsion angles in eglin c. Eur. J. Biochem. 164, 625–635 (1987).
Cavanagh, J., Chazin, W. & Rance, M. The time dependence of coherence transfer in homonuclear isotropic mixing experiments. J. magn. Reson. 87, 110–131 (1990).
Zuiderweg, E.R.P., Boelens, R. & Kaptein, R. Stereospecific assignments of 1H-NMR methyl lines and conformation of valyl residues in the lac repressor headpiece. Biopolymers 24, 601–611 (1985).
Bartik, K. & Redfield, C. A method for the estimation of χ1 torsion angles in proteins. J. biol. NMR 3, 415–428 (1993).
Koning, T.M.G., Boelens, R. & Kaptein, R. Calculation of the nuclear Overhauser effect and the determination of proton-proton distances in the presence of internal motions. J. magn. Reson. 90, 111–123 (1990).
Nilges, M., Gronenborn, A.M., Brünger, A.T. & Clore, G.M. Determination of three-dimensional structures of proteins by simulated annealing with interproton distance restraints. Application to crambin, potato carboxypeptidase inhibitor and barley serine protease inhibitor 2. Prot. Engng. 2, 27–38 (1988).
Brünger, A.T. X-PLOR manual. Version 3.0 (Yale University, New Haven, Connecticut, 1992).
Hyberts, S.G., Goldberg, M.S., Havel, T.F. & Wagner, G. The solution structure of eglin c based on measurements of many NOEs and coupling constants and its comparison with X-ray structures. Prot. Sci. 1, 736–751 (1992).
Brooks, B.R. et al. CHARMM: a program for macromolecular energy, minimization and dynamics calculations. J. comput. Chem. 4, 187–217 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baumann, H., Knapp, S., Lundbäck, T. et al. Solution structure and DNA-binding properties of a thermostable protein from the archaeon Sulfolobus solfataricus. Nat Struct Mol Biol 1, 808–819 (1994). https://doi.org/10.1038/nsb1194-808
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1194-808
This article is cited by
-
Growth temperature and chromatinization in archaea
Nature Microbiology (2022)
-
Two Approaches to Enhance the Processivity and Salt Tolerance of Staphylococcus aureus DNA Polymerase
The Protein Journal (2019)
-
Beyond antibody engineering: directed evolution of alternative binding scaffolds and enzymes using yeast surface display
Microbial Cell Factories (2018)
-
Identification and characterization of a novel Sso7d scaffold-based binder against Notch1
Scientific Reports (2017)
-
The archaeal “7 kDa DNA-binding” proteins: extended characterization of an old gifted family
Scientific Reports (2016)